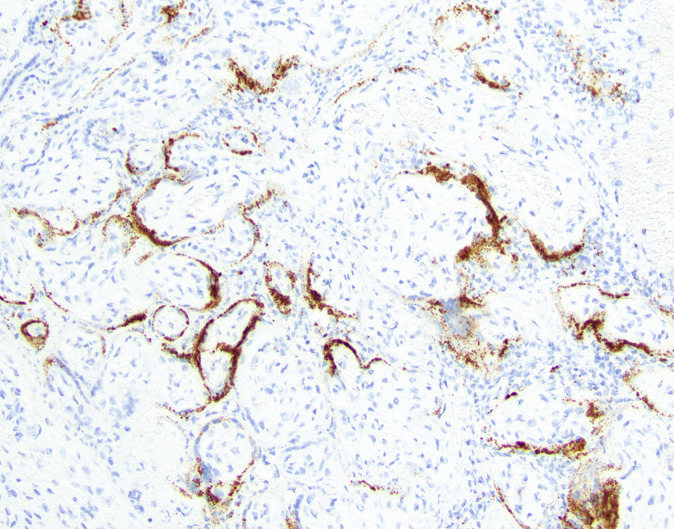
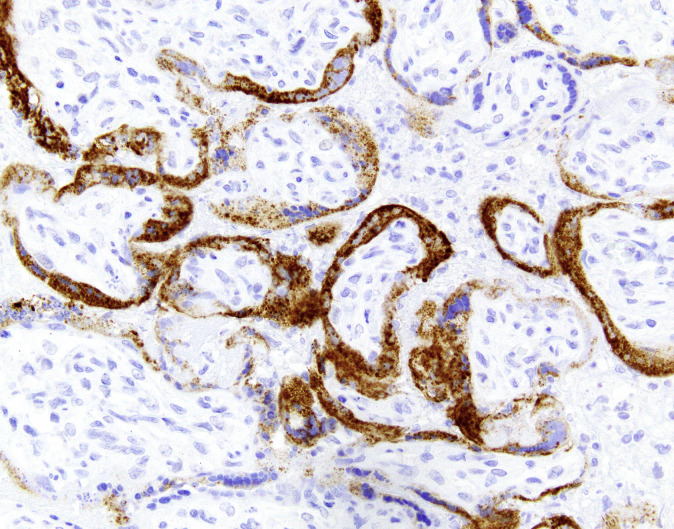
Table 2.
Definition specifics of placental infection with SARS-CoV-2 in order of rigor
| Definite: documentation of viral presence, location in the placenta tissues, and replication, by: |
|
| or |
|
| Probable: documentation of viral proteins or RNA within placental tissues, without evidence of active replication via: |
|
| or |
|
| Possible: less specific detection of virus. These approaches could be detecting viral particles engulfed by macrophages rather than actively replicating virus. RT-PCR of placental homogenates theoretically may have a positive result owing to maternal viremia (although this is a rare entity), rather than placental involvement. |
|
| Note that an alternative approach is a 2-step approach, in which RT-PCR is used as a screen and then followed up with one of the methods recommended to confirm “definite” or “probable” infection. This hybrid/2-step approach would be more rigorous than RT-PCR alone and potentially more sensitive than the “definite” and “probable” approaches. |
|
| Unlikely: Negative results from any of the above tests |
| No testing: placenta not tested |
PBS, phosphate-buffered saline; RNA-ISH, RNA in situ hybridization; RT-PCR, reverse transcription polymerase chain reaction.
Roberts et al. Consensus definition of SARS-CoV-2 placental infection. Am J Obstet Gynecol 2021.