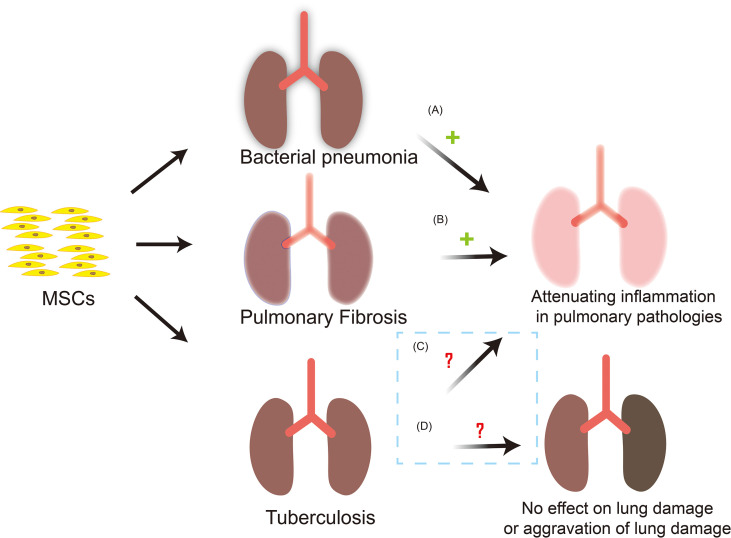
Figure 3.
MSCs-based clinical application and possible outcomes for TB. MSCs are used in the treatment of bacterial pneumonia and PF, and have achieved good results. Nevertheless, the safety and effectiveness of MSCs in the treatment of tuberculosis need to be further confirmed. (A) MSCs can reduce the severity of bacterial pneumonia, reduce the bacterial load in the lungs and inhibits inflammation, and reduce lung injury. (B) After MSCs infusion, there are fewer inflammatory cells in the lung interstitium and collagen fibers. The deposition in lung tissue is reduced and the degree of pulmonary fibrosis is reduced. (C) MSCs infusion can effectively reduce the inflammatory response in vivo, hinder the development of fibrosis in the lesion site, and does improve the prognosis of TB patients to a large extent. However, the safety and effectiveness need to be verified. (D) During Mtb infection, MSCs may not be able to effectively regulate the local immune response and the course of disease. In addition, MSCs have immunosuppressive properties, and its infusion may have the risk of worsening of TB or the reactivation of latent Mtb, so the safety and effectiveness of MSCs in the treatment of TB are issues worthy of in-depth exploration. +: outcomes are effective; ?: outcomes are uncertain.