FIGURE 1.
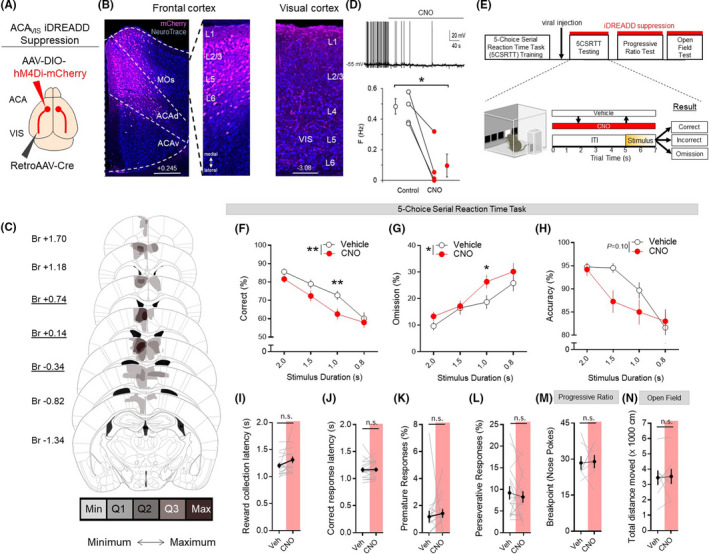
Chemogenetic suppression of ACAVIS neuron activity disrupts attentional behavior. A, Intersectional viral strategy: To selectively express iDREADD in ACAVIS neurons, Cre‐dependent iDREADD‐mCherry and retrograde Cre encoding AAVs were injected bilaterally into the ACA and VIS, respectively. B, Representative images of iDREADD (pink)‐expressing ACAVIS neurons and NeuroTrace (blue) in both dorsal ACA (ACAd) and secondary motor cortex (MOs) of frontal cortex (left, Scale bar = 200 μm) and axon terminals in the visual cortex (right, Scale bar = 100 μm). C, Histological verification of ACAVIS iDREADD viral expression location within the frontal cortex. Underlined distance from bregma (Br, mm) indicates injection location. Min: at least one mouse had viral expression in this area. Q1: >25% of mice had expression in this area. Q2: >50% of mice had expression in this area. Q3: >75% of mice had expression in this area. Max: 100% of mice had expression in this area. D, Electrophysiological validation of iDREADD in ACAVIS neurons. Top: Representative trace of whole‐cell patch recording from ACAVIS neuron in frontal cortex slice upon bath application of CNO. Bottom: CNO significantly decreased firing frequency of ACAVIS neurons expressing iDREADD during whole‐cell recording in slice (two‐tailed paired t test, t 3 = 5.09, *P = .0147, n = 3 mice, four cells). E, Experimental timeline: Mice were first trained on the 5CSRTT before viral injection. After allowing three weeks for maximal viral expression, mice underwent 5CSRTT testing. Mice were treated with saline (vehicle) or clozapine‐N‐oxide (CNO, 10 mg/kg) 30 min prior to testing in a counterbalanced manner with a fixed 5‐s intertrial interval (ITI) and pseudorandomized stimulus duration (2.0, 1.5, 1.0, or 0.8 s; n = 16 mice; 4944 total trials). F‐H, ACAVIS neuron activity suppression via acute CNO administration significantly decreased correct trials (%, two‐way repeated measures analysis of variance, (RM ANOVA), F 1,15 = 10.03, **P = .0064, Holm‐Sidak multiple comparisons at 2.0, 1.5, 1.0, and 0.8 s stimulus duration, P = .6028, .1575, **.008, .9443, n = 16 mice) and omissions (two‐way RM ANOVA, F 1,15 = 5.341, *P = .0344; Holm‐Sidak multiple comparisons at 2, 1.5, 1.0, and 0.8 s, P = .5797, .9980, *.0325, .4172, n = 16 mice), but had no significant yet trending effect on accuracy (two‐way RM ANOVA F 1,15 = 3.038, P = .10, n = 16 mice). I‐L, ACAVIS neuron suppression had no effect on reward collection latency (t 15 = 1.770, P = .0970), correct response latency (t 15 = 0.3928, P = .7000), premature responses (t 15 = 0.8159, P = .4273), and perseverative responses (t 15 = 0.6747, P = .5101) during 5CSRTT testing (n = 16 mice). M, Acute CNO administration had no effect on motivation as independently measured using a progressive ratio task (t 6 = 0.1448, P = .8896, n = 7 mice). N, Acute CNO administration had no effect on motor activity as independently measured during open field testing (t 7 = 0.1959, P = .8502, n = 8 mice). I‐L, Two‐tailed paired t test. Error bars indicate mean ± SEM, n.s. = nonsignificant, *P < .05, **P < .01. Data available in Table S1
