Abstract
Obstructive sleep apnea (OSA) is a complex disorder characterized by collapse of the upper airway during sleep. Downstream effects involve the cardiovascular, pulmonary, and neurocognitive systems. OSA is more prevalent in men than women. Clinical symptoms suggest the diagnosis of OSA but none is pathognomonic of the condition. With rising awareness of OSA and the increasing prevalence of obesity, OSA is increasingly recognized as a major contributor to cardiovascular morbidity including systemic and pulmonary arterial hypertension, heart failure, acute coronary syndromes, atrial fibrillation, and other arrhythmias. Pulmonary manifestations include the development of chronic thromboembolic disease, which can then lead to chronic thromboembolic pulmonary hypertension (CTEPH). Neurocognitive morbidities include stroke and neurobehavioral disorders. Screening for OSA includes the use of symptom questionnaires and the diagnosis is confirmed by polysomnography. Management primarily includes the use of continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP) devices during sleep. Alternate options such as mandibular devices and surgical procedures are considered for certain patient populations.
Keywords: Sleep Apnea, Obstructive, Airway Obstruction, Sleep Apnea Syndromes
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder caused by upper airway obstruction (which can be partial or complete) during sleep1. The change in airway muscle tone during sleep leads to collapse of the upper airways (predominantly during the inspiratory phase of breathing), which leads to intermittent episodes of hypopnea and/or apnea2,3. During these episodes the arterial oxygen saturation falls, which can lead to autonomic dysregulation3. These acute changes result over time in chronic conditions that affect the cardiovascular, pulmonary, and neurocognitive systems2,3. In 1956, Bickelmann et al.4 described obesity hypoventilation syndrome (OHS) in a report regarding an obese business executive who presented to the hospital complaining of excessive daytime sleepiness. These investigators attributed the name “Pickwickian syndrome” to the condition based on the description of a similar fictional character in Charles Dickens’ first novel (1836-37) “The posthumous papers of the Pickwick Club”4. Following that, numerous descriptions of the obesity hypoventilation syndrome, central sleep apnea and obstructive sleep apnea were published under the rubric of sleep- disordered breathing (SDB).
Currently, obstructive sleep apnea (OSA) is the most prevalent, clinically significant SDB, and it is known to be associated with numerous diseases including hypertension, atrial fibrillation, heart failure, cerebrovascular accidents, pulmonary hypertension and others2,5,6. The goal of this article is to compile and therefore understand the impact OSA has on other organ systems.
Prevalence and risk factors
OSA is more prevalent in men than women1,2. Benjafield et al.7 performed an extensive review to gauge the worldwide prevalence of this disease, which consisted of reliable prevalence data from 16 countries including Brazil, Germany, Spain, China, Switzerland, and USA. The worldwide prevalence of OSA was extrapolated from this data and showed that about 1 billion people aged 30-65 years are affected by OSA, 425 million of those deemed to have moderate to severe OSA7. The prevalence of SDB among 30-49-year-old men in North America is 10% compared to 3% among women in the same age bracket, and 17% among 50-70-year-old men, compared to 9% among women in the same age bracket3.
Numerous risk factors are associated with OSA that can be detected on physical examination.
Obesity and high body mass index are the strongest risk factors predisposing OSA. There is a linear correlation between OSA and obesity1,6;
Neck circumference greater than 17 inches (43cm) in men and 15 inches (38cm) in women8;
Male gender1;
Other risk factors include menopause, neuropathy or myopathy that may affect the upper airway muscles (particularly the genioglossus muscle), craniofacial anatomical structure (particularly in the Asian population), family history, smoking, and nasal congestion2,5,8.
Patients with acromegaly may have OSA due to macroglossia and they develop central sleep apnea due to altered respiratory control9.
Clinical symptoms
Clinical symptoms play a key role in identifying patients with OSA but none is pathognomonic of the disease.
Patients usually complain of fatigue, excessive daytime sleepiness, snoring, drooling, nocturnal gasping or choking, headaches and/or falling asleep while driving10. Patients with OSA are more likely to be involved in motor vehicle collisions10.
The purpose of this article is to review the prevalence, risk factors, clinical presentation, effects of OSA on different organ systems, diagnostic criteria, and to discuss current approaches to the management of patients with OSA.
OSA & cardiovascular disease
OSA is well recognized as a major contributor of cardiovascular disease. Previous studies have not definitively identified the direct link between OSA and cardiovascular disease, since patients with OSA often have other risk factors for cardiovascular disease such as hypertension (HTN), obesity, diabetes, and smoking. Theoretical explanations of the association of OSA and cardiovascular disease include the observations that OSA produces a chronic inflammatory state, which leads to increased atherosclerotic changes in the blood vessels of the patient.
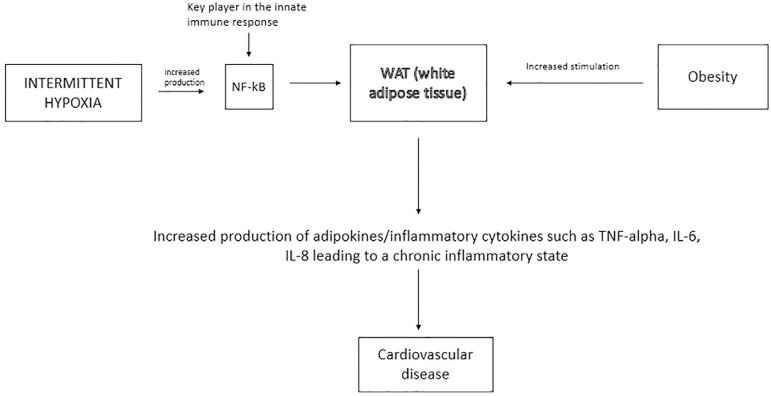
Similar to obesity, which is also considered a low-grade inflammatory state, OSA has been shown to stimulate the white adipose tissue (WAT) leading to the production of inflammatory mediators11. OSA leads to sleep fragmentation, intermittent hypoxemia and in some instances, recurrent hypercapnia, which in turn, stimulate increased sympathetic activity, increased systemic inflammation, and increased oxidative stress. The end result of these changes is endothelial dysfunction and metabolic dysfunction which accounts for the increase in cardiovascular disease. Among the aforementioned factors, intermittent hypoxemia has been shown to play a critical role by promoting increased production of inflammatory markers, as depicted in Figure 1 11-14.
Figure 1.
Pathogenesis of cardiovascular disease secondary to OSA.
OSA & systemic hypertension
Evidence has been growing steadily for systemic arterial hypertension (HTN) and OSA as cardiovascular disease risk factors. In 1980, Lugaresi et al.15 associated systemic hypertension with snoring in the general population. In 1985, Fletcher et al.16 evaluated forty-six middle aged/older men with essential hypertension and thirty-four normotensive, age and weight matched controls for undiagnosed sleep apnea. Thirteen in the study group and three in the control group were found to have undiagnosed sleep apnea. Seven men with hypertension and sleep apnea were treated with protriptyline and one underwent uvulopalatopharyngoplasty (UPPP). A reduction in mean blood pressure (BP) was observed (149/95mmHg to 139/90mmHg) accompanied by a significant decrease in the apnea-hypopnea index (AHI) by 77%. The investigators concluded that OSA could be either the cause or a contributor to systemic arterial hypertension16.
Due to the prevalence of obesity as a confounding factor among studied patient populations, the confirmation of the association between OSA and HTN has been challenging17,18. However, more recently association of nocturnal OSA and daytime hypertension has been demonstrated, even after the adjustment for body mass index19-21. A prospective longitudinal cohort study with 1889 participants followed for 12.2 years (median) years identified an independent association of OSA and HTN from the confounders including age and obesity. The study demonstrated an increased hazard ratio for incident HTN in patients with OSA compared to the control subjects. Further follow up revealed a dose-response relationship between the severity of OSA and the cumulative incidence of HTN22.
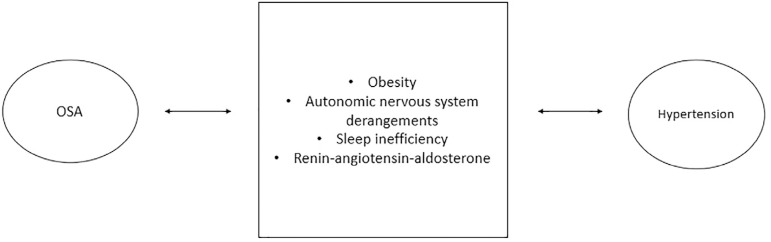
The pathophysiology of the association between OSA and HTN is multifactorial. The potential causative factors are summarized in Figure 2.
Figure 2.
Association of OSA and hypertension.
Obesity has been identified as an independent risk factor both for OSA and HTN22. Adipose tissue deposition in the oropharyngeal around the upper airway contributes to apnea.
Downstream effects of OSA include physical inactivity, poor dietary habits, insulin resistance, hyperleptinemia, and systemic inflammation. The vicious cycle between obesity and apnea exacerbates HTN23-26. The nocturnal dipping pattern of BP is a normal physiological phenomenon that is affected by OSA27. Intermittent hypoxia and hypercapnia cause autonomic derangements which lead to nighttime increases in BP, and increased catecholamine levels which persist during daytime, worsening HTN28. OSA is well known to cause sleep inefficiency, which in itself has been shown to be correlated with several cardiovascular risk factors such as non-dipping of nocturnal BP, endothelial dysfunction, arterial stiffness, and increased sympathetic activity. The renin-angiotensin system (RAS) is known to be activated by obesity and more recently, it is known to be activated by OSA. The presence of OSA has a cumulative effect on obesity-induced activation of RAS, thereby worsening HTN29.
Phillips et al performed a randomized controlled trial to compare the change in cardiovascular and neurobehavioral outcomes among patients using CPAP and mandibular advancement devices (MAD). They found that although CPAP was more effective at reducing AHI, patient compliance was better with MADs. They also found that neither treatment improved blood pressure30. However, the meta-analysis by Montesi et al.31 demonstrated a significant reduction in the systolic and diastolic blood pressure of patients when treated with CPAP for their OSA. Reduced nighttime BP and sympathetic traffic can be achieved with effective OSA treatment resulting in more successful BP control31.
OSA & heart failure
Sleep-disordered breathing (SDB), (central sleep apnea [CSA] and OSA), was found to be more prevalent in patients with heart failure (HF)32. However, OSA often remains as an undiagnosed risk and contributing factor for heart failure. Paulino et al.33 demonstrated that the prevalence of sleep breathing disorder was 81% (n=256) in 316 patients, 30% of whom were classified as CSA and 70% as OSA. Among this cohort of patients, both CSA and OSA existed together due to certain pathophysiological changes brought on by heart failure leading to SDB.
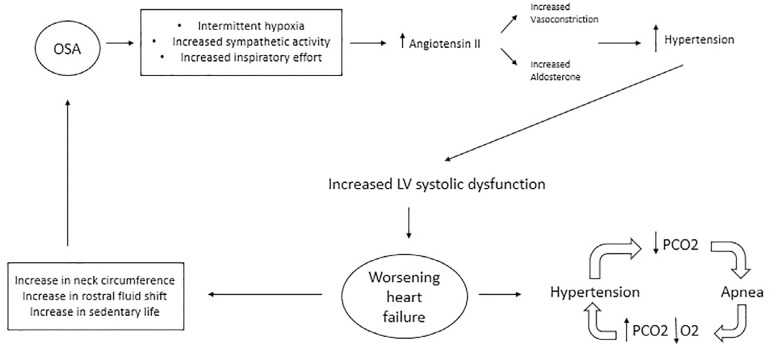
The interactions of the heart failure and OSA are illustrated in Figure 3, OSA can lead to intermittent hypoxemia, which activates inflammatory pathways and promotes oxidative stress. Increased inspiratory effort against the high resistance of the upper airway leads to increased arousals and, combined with intermittent hypoxia, leads to sympathetic activation11. In addition, the high negative intrathoracic pressure exerted during inspiration through a narrowed or occluded upper airway may increase pulmonary capillary fluid efflux contributing to interstitial edema.
Figure 3.
Association of OSA and heart failure.
Increased sympathetic activity leads to increased angiotensin II release which is a potent vasoconstrictor promoting aldosterone production via the adrenal cortex. Aldosterone increases water and salt reabsorption leading to increased intravascular volume, worsening HTN and heart failure34. Chronic untreated OSA can lead to persistently elevated BP with absent nighttime dipping of BP, contributing to left ventricular systolic dysfunction35.
There are varied mechanisms by which heart failure may worsen OSA and induce apnea. Fluid overload leading to upper airway (UA) narrowing and upper airway edema are important mechanisms leading to worsening OSA36. In systolic heart failure patients, nighttime rostral fluid shift from the legs is a contributing pathophysiological mechanism causing worsening of OSA in HF37. Overnight rostral leg fluid displacement and an increase in neck circumference have been shown to significantly worsen OSA and CSA. There is a positive correlation with the volume of lower extremity extravascular fluid volume37. The brain stem respiratory centers in the medulla and pons receive input from peripheral arteries and the respiratory system. The effectors for the ventilatory center are the respiratory muscles. Heart failure leads to hypoxemia and increased afferent activity from the juxta capillary receptors (due to pulmonary capillary engorgement) which in turn drives the ventilatory center leading to hyperventilation38. Hyperventilation leads to decreased PaCO2 levels, resulting in hypopnea and/or apnea.
Also, increased circulation time leads to slower feedback from the chemoreceptors in the peripheral arteries. Pulmonary vascular congestion and edema lead to lower alveolar PAO2 and PACO2 reserve adds to ventilatory instability39.
OSA and acute coronary syndrome
OSA is prevalent in patients with preexisting cardiovascular diseases. The plausible factors contributing to the development and maintenance of cardiovascular impairment in patients with OSA include intermittent hypoxemia, development of acidosis, increase in blood pressure and sympathetic vasoconstriction11.
The prospective sleep heart health study (SHHS)40 was done to establish the association between OSA and incident coronary artery disease and heart failure. For the purposes of data collection and analysis regarding incident coronary disease, the first occurrence of myocardial infarction, coronary heart disease (CHD), death, or coronary revascularization procedures were included. The rate of incident CHD was 20.1 events per 1000 person-years of follow-up in men and 8.7 events per 1,000 person-years in women. Event rates increased with severity of OSA in men. When these models were adjusted for age, race, BMI, and smoking status, there was a significant association of apnea/hypopnea index (AHI) with incident CHD in men but not in women. However, this association was not statistically significant after accounting for diabetes mellitus and lipid measures, adjustment for systolic and diastolic blood pressure and anti-hypertensive medication use also diminished the significance of the association.
Other prospective studies have shown an increased association between OSA and CHD. Shah et al.41 assessed whether OSA increased the risk of cardiovascular events. The outcomes studied included MI, coronary artery revascularization procedures and death from cardiovascular causes. These investigators found that patients with OSA had an increased risk of these outcomes, despite controlling for other cardiovascular risk factors including diabetes, hypertension, hyperlipidemia, tobacco, and alcohol use. A systematic review by Porto et al.42 supported an association between OSA and MI, which was greater in men.
Another observational study compared cardiovascular outcomes (fatal and non-fatal) in men with OSA who were being treated with CPAP versus untreated men with OSA43. Fatal myocardial infarction (MI) and stroke, non-fatal MI, non-fatal stroke, coronary artery bypass surgery, and coronary angiography were the study parameters. Severe OSA significantly increased the risk of fatal and non-fatal cardiovascular outcomes. The study also demonstrated that CPAP treatment in patients with severe OSA reduced the aforementioned adverse outcomes and those patients with mild and moderate OSA, did not exhibit increased risk of these outcomes. Buchner et al.44 reported that OSA treatment with CPAP had a benefit for patients with all severities of OSA and resulted reduced adverse cardiovascular outcomes. Interestingly the SAVE (sleep apnea cardiovascular endpoints) study45, a randomized control trial conducted to assess the effects of CPAP on major cardiovascular events, showed that CPAP use did not prevent cardiovascular events in patients with moderate to severe sleep apnea. It did reduce snoring and daytime sleepiness and improved health-related quality of life and mood. Interestingly the observational study conducted by Anandam et al.46 showed that CPAPs and MADs may have similar effectiveness in reducing cardiovascular mortality.
OSA and atrial fibrillation
Atrial fibrillation (AF) is the most common arrhythmia linked with OSA. The prevalence of AF in patients with known OSA is 5%47. OSA may trigger the onset of and contributes to its persistence48. However, OSA is more common and less frequently detected in patients with AF47,49. The severity of OSA correlated with a higher incidence of AF and may also be a predictor of AF recurrence after cardioversion and/or ablation procedures50,51. There is limited success of antiarrhythmic therapy in patients with severe OSA52. Patients with OSA are more likely to develop AF post coronary artery by-pass graft surgery (CABG)53. OSA is associated with an increased incidence of AF in patients with heart failure54, coronary artery disease (CAD), and hypertrophic cardiomyopathy (HCM)55.
Pathophysiology of developing AF in patients with OSA
Mechanisms linked to the development of AF in patients with OSA include the hemodynamic changes occurring during the apneic episodes. Hypoxemia and hypercapnia during episodes of hypopnea/apnea leads to tachycardia and hypertension. These changes are accompanied by increased myocardial oxygen demand, despite the restricted supply of oxygen during these episodes. This leads to myocardial injury and fibrosis, which promote the development of AF11,47. Episodes of hypopnea/apnea and post apneic reoxygenation also lead to oxidative stress, which contributes to the remodeling of the myocardium47. OSA is associated with higher levels of CRP and IL-656, however the use of CPAP therapy has been shown to decrease these inflammatory markers in patients with OSA56.
OSA is also associated with atrial enlargement, conduction abnormalities, and prolonged sinus node recovery time57. This structural and electrical remodeling contributes to the development of AF.
Negative intrathoracic pressure during apneic episodes may be associated with the development of AF. The Mueller maneuver was performed on healthy adults to simulate the changes in the upper airway, which occur during OSA58 and found that the negative intrathoracic pressure led to a decrease in the left atrial volume, increase in left ventricular systolic function and an increase in the ventricular afterload. These dynamic changes can be implicated in the development of AF. Repetitive cycles of negative intrathoracic pressure also lead to atrial stretch, which contributes to the enlargement of the chambers and development of AF48. Negative intratracheal pressure causes shortening of the atrial effective refractory period through vagal stimulation, which also predisposes to the development of AF59.
Management of AF in OSA
OSA is considered a modifiable risk factor for AF47. Current evidence suggests that continuous positive airway pressure is the standard treatment for OSA. Shah et al.60 showed that CPAP had a beneficial effect on left ventricular remodeling. Use of CPAP is associated with effective lowering of blood pressure, decreased atrial size and ventricular mass, and a lower risk for AF recurrence after ablation61. Recurrence of AF after cardioversion is also less frequent in patients being treated with CPAP compared to patients not being treated with62. Bayir et al.63 showed that after 6 months of therapy with CPAP in patients with OSA, there was an improvement in the interatrial, left intra-atrial and right intra-atrial electromechanical delays when compared to pretreatment measurements and possibly a decreased risk for OSA related AF63. CPAP can also help reverse left atrial volumetric abnormalities in as little as 12 weeks and improve left atrial remodeling over a period of 24 weeks64. Furthermore, it reduces the risk of progression from paroxysmal AF to persistent AF65. Drug refractory AF is often treated with catheter ablation (or a convergent procedure); however, screening patients with atrial fibrillation for OSA prior to catheter ablation may be beneficial, and could possibly obviate the need for the procedure66.
Renal nerve denervation (RND) is an emerging modality that may be of benefit66. Despite the failure of RND in managing drug resistant hypertension, there are new experimental studies suggesting that it may control arrhythmias caused by hyperactivity of the sympathetic nervous system67,68. Linz et al.68 studied the effects of RND on anesthetized pigs and determined that RND reduced AF caused by negative intratracheal pressure and reduced shortening of the atrial effective refractory period, in contrast to administration of atenolol which did achieve the same results68. They also reported that RND prevented post apneic elevation in blood pressure, decreased plasma renin activity and aldosterone levels68.
OSA and other arrhythmias
OSA is associated other arrhythmias including ventricular tachycardia, premature ventricular contractions, ventricular fibrillation, sinus bradycardia and sick sinus syndrome (SSS)69,70.
OSA is also associated with bradycardia, long pauses and sick sinus syndrome. Simantirakis et al.71 conducted a study on 23 patients with an established diagnosis of OSA to evaluate their arrhythmias and to determine the effect of CPAP therapy on those arrhythmias. These investigators noted that these patients had multiple episodes of bradycardia and long pauses during sleep. Their study also showed that treatment with CPAP reduced these episodes. In another report, the prevalence of SSS was 31.6% in patients with OSA72.
Abe et al.73 performed a study on 1,394 Japanese patients and found that CPAP therapy substantially reduced the incidence of AF, PVCs, sinus bradycardia, and sinus pauses.
OSA and pulmonary hypertension
Pulmonary hypertension (PH) is frequently associated with OSA, and often is a direct consequence of OSA74. The prevalence of PH in OSA ranges from 17 to 53%74. According the updated guidelines from 6th World Symposium on PH (March, 2019), PH is defined by a mean pulmonary artery pressure (mPAP) >20mmHg rather than >25mmHg, and a pulmonary vascular resistance (PVR) >3 wood units is included to distinguish those with pre-capillary PH75.
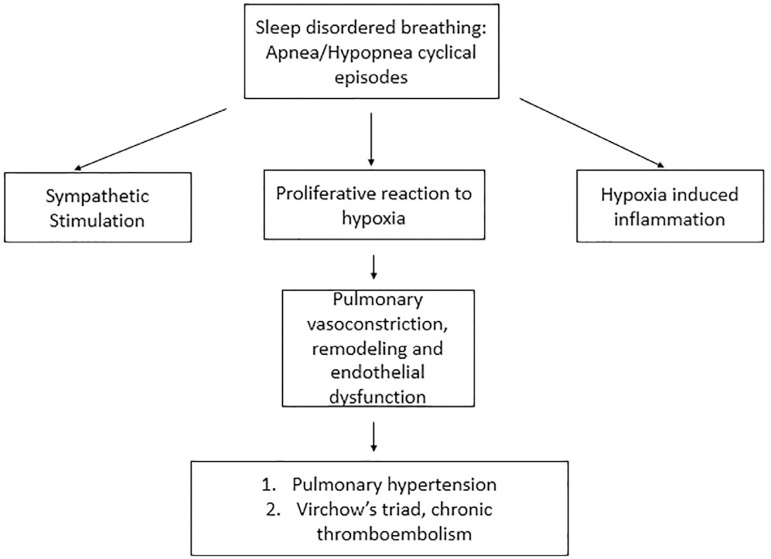
Figure 4 summarizes the three main factors believed responsible for the increase in the PAP observed in sleep apnea:
Figure 4.
Association of OSA and pulmonary vasculature.
Alveolar hypoxia, causing pulmonary vasoconstriction and endothelial remodeling;
Mechanical related to increased inspiratory effort resulting in, more negative intrathoracic pressure, variations in heart rate and cardiac output, increased left heart filling pressures;
Reflex mechanisms directly influencing the vasculature76.
Acute pulmonary artery pressure changes during sleep have been reported with obstructive apneas76. However, the role of OSA as an independent risk factor for the development of daytime PH is not fully established. Severe OSA frequently causes daytime PH in the absence of significant co-existing cardiopulmonary and vascular diseases, and CPAP therapy significantly reduces the levels of daytime pulmonary artery pressure77.
Daytime PH may have a precapillary component related to repetitive hypoxia-reoxygenation78 leading to both pulmonary vasoconstriction and vascular endothelial remodeling, but there may also be a post capillary component attributable to episodic or permanent elevations in LV filling pressure79. The application of nocturnal CPAP therapy can lead to the abolition of both nocturnal hypoxemia and the accompanying sympathetic surges, resulting in improvement in LV diastolic relaxation and decreased LV afterload. This may promote the restoration of the balance among these endothelial vasoactive mediators. Long-term CPAP therapy may avoid irreversible structural pulmonary vascular and right ventricle changes by reducing the pulmonary artery systolic pressure, possibly improving the prognosis77.
OSA and CTEPH
OSA promotes the release of inflammatory markers56 and activates the coagulation cascade, which increase the risk of acute thromboembolic events, and also predisposes patients to the development of Type 4 CTEPH (chronic thromboembolic pulmonary hypertension)80.
Evidence supports the pathophysiological association of sleep-disordered breathing as an independent risk factor for venous thromboembolism. There is increased prevalence of sleep apnea in patients with acute pulmonary embolism and/or deep vein thrombosis80,81.
Obstructive sleep apnea-related hemodynamic alterations may result in venous stasis, increased thrombogenicity (on a vascular and molecular level), increased inflammatory insult and injury, thus fulfilling the criteria for the nomenclature of Virchow’s triad82.
The development of CTEPH may be promoted by the persistence of thrombotic material in the circulation, stemming from inadequate/incomplete thrombus resolution82,83. Chronic hypoxia and hypercapnia in OSA impair thrombus resolution due to inadequate fibrinolysis, persistent inflammation, vascular smooth muscle activation, accelerated adhesion molecule expression and platelet activation82-84.
OSA and cerebrovascular accidents
Obstructive sleep apnea has been associated with hypoxic-ischemic brain injury (HI-BI), the severity of which depends on the duration and intensity of hypoxemia and ischemia85. EEG microarousals and awakenings frequently follow respiratory compromise. Repeated oxyhemoglobin desaturation causes alterations in sympathetic nerve activity, oxidative stress, inflammatory markers and endothelial function, which are associated with decreased vasoreactivity, increased arterial wall stiffness, increased platelet activation and vascular adhesion, resulting in increased risk for cardiovascular and cerebrovascular insults.
Numerous studies have shown the association between OSA and stroke86,87. A systematic review of 37 studies with 3,242 patients showed a high prevalence of OSA in patients with cerebrovascular disease (61.9%)88. A meta-analysis of 16 cohort studies reporting data on 24,308 patients demonstrated that moderate and severe OSA are associated with an increased risk of vascular outcomes, including stroke11. Patients with OSA are more likely to have a stroke or die than those without OSA87. There is an association between OSA and nocturnal cerebrovascular events89-91, and a dose-effect relationship has been described between the adjusted risk and OSA severity, as measured by AHI and oxygen desaturation index, viz., the mean number of desaturations of 4% or more per hour of sleep86. OSA contributes to ischemic stroke both as a predisposing risk factor and as a triggering factor; there is a statistically significant association between preceding OSA symptoms and wake-up stroke (WUS)92. A higher percentage of cerebral white matter disease, radiographic deep grey matter disease or macroangiopathic strokes is noted in individuals with OSA89. A study of 61 patients with silent cerebral infarct and 122 without silent lacunar cerebral infarct demonstrated that the presence of severe OSA syndrome was significantly higher in silent cerebral infarct in comparison to patients without lacunar infarcts (55.8% versus 35.7%, p=0.019)93. Fluctuations in cerebral blood flow velocity (CBFV) have been documented in OSA, with CBFV increasing along with arterial pressures during OSA episodes. Both CBFV and systemic arterial pressures decrease upon termination of the apneic episode, at the lowest level of oxyhemoglobin desaturation94.
Transcranial Doppler imaging has shown decreased cerebrovascular reactivity and increased arterial stiffness, particularly during OSA episodes95. An impairment in cerebral autoregulation by means of measurement of the recovery of CBFV and cerebrovascular conductance (CBFV/mean arterial pressure) has been observed after orthostatic challenges, with slower recovery noted in patients with OSA compared to control subjects96. A dose-relationship between severity of sleep respiratory disturbance (as measured by AHI) and impaired cerebral autoregulation has also been noted97. An impairment in cerebrovascular CO2 reactivity, measured by the CBF with increasing AHI was demonstrated in one case control study98. Global cerebral blood flow increases during episodes of hypoxemia. A study comparing the increase in CBF in patients with OSA compared to that in healthy subjects demonstrated less increase CBF in OSA patients compared to the control subjects, and this difference was not demonstrable after 3 months of treatment with CPAP99. Data from other studies suggest that it is possible to normalize cerebral vasoreactivity and cerebral blood flow with CPAP treatment100,101.
Functional outcomes and long-term mortality of stroke patients with OSA are poor compared to those without OSA. Patients with a higher AHI required longer inpatient rehabilitation and had lower Functional Independence Measure (FIM) scores102. Increased mortality 60 months after stroke was observed in those with higher AHI89. The nocturnal nadir of oxyhemoglobin saturation is an independent predictor of poor functional outcomes103. One study demonstrated that higher severity of SDB correlated with a poorer functional outcome based on the modified Rankin scale score104.
There have been concerns about the safety CPAP treatment in patients of acute stroke occurring in the setting of OSA. It is thought that CPAP treatment may reduce cerebral perfusion by altering blood oxygen and carbon dioxide balance. Despite these concerns, current data obtained from prospective and cohort studies suggest no adverse effect of CPAP treatment in patients with OSA during acute stroke89,105.
The effect of continuous positive airway pressure (CPAP) treatment was evaluated as a primary outcome measure for prevention of new vascular events among OSA patients with stroke. Concurrently, secondary outcome measures were designed to assess post stroke clinical outcomes utilizing the Barthel index and the modified Rankin scale. Measurement of neuropsychological parameters suggested better stroke outcomes and there was a trend toward favorable outcomes vis-a-vis reduced recurrence of vascular events35. A meta-analysis of seven randomized controlled trials which included 4,268 patients showed a significant reduction in relative risk or major adverse cardiovascular events and stroke, which correlated with increased CPAP usage time (adherence time >4 hours)106. Another systematic review and meta-analysis of 4 randomized clinical trials and 1 prospectively matched observational cohort, (total of 389 patients) showed a mean decrease in National Institutes of Health Stroke Scale scores during the first (≤30) days of acute ischemic stroke in patients treated with non-invasive ventilation (NIV) compared to control subjects (standardized mean difference, 0.38; 95% confidence interval, 0.11-0.66; p=0.007)107.
Although several studies have demonstrated the beneficial effect of CPAP on recovery outcomes in stroke patients, including more rapid functional recovery, reduced hospitalization time, and decreased frequency of re-hospitalization, significant challenges remain due to post stroke disability which may lead to limited CPAP adherence in the hospital environment.
Consequently, there has been increased interest in alternate options to treat SDB such as mandibular advancement surgery and supine avoidance. Additional studies are needed to evaluate their efficacy in post-stroke rehabilitation outcomes108.
OSA and other neurological disorders
OSA increased the risk of developing optic neuropathy after controlling for comorbidities as demonstrated in a Taiwanese population-based cohort study; however, treatment with CPAP did not reduce the risk of optic neuropathy109.
Review performed by Chaitanya et al.110 highlights OSA as a risk factor for developing glaucoma. Another important point to note is that CPAP therapy can trigger glaucoma damage by raising the intraocular pressure (IOP), which would warrant glaucoma screening in patients on CPAP.
A study to investigate the association between obstructive sleep apnea (OSA) and middle ear acoustic transference/cochlear function demonstrated that severe OSA is associated with cochlear function impairment in patients. Patients with severe OSA presented with significantly lower distortion product otoacoustic emissions (DPOAE) amplitudes when compared to the control, mild, and moderate OSA groups111.
Fecal and urinary incontinence has been reported to resolve after treatment with positive airway pressure non-invasive ventilation112 in a patient with obstructive sleep apnea hypopnea syndrome (OSAHS). Five adults OSA patients who presented with enuresis, enuresis resolved after treatment with continuous positive airway pressure (CPAP)113, and similar improvement was noted in another case report114.
Patients with OSA demonstrate impairments in behavior, cognition, and physical skills85. Excessive daytime sleepiness, as measured by the subjective Epworth sleepiness scale (ESS) and the objective multiple sleep latency test (MSLT) and the maintenance of wakefulness test (MWT) is the most common neurobehavioral consequence of OSA. Other behavioral problems occurring in this patient population include disinhibition, distractibility, and irritability115,116. Physical skills and cognitive abilities, including selective attention, vigilance, short-term and working memory, and executive and motor functioning are adversely affected by OSA115-121. Most neurobehavioral deficits, except executive dysfunction, have been found to be reversible with CPAP treatment of OSA118,122-124. CPAP treatment for as little as 2 weeks has been shown to improve daytime sleepiness, including reduction in subjective sleepiness as measured by the ESS, but no significant changes were observed in objective sleepiness measured by MSLT or MWT125-127. Several key neurobehavioral indices (functional outcomes of sleep questionnaire, Epworth sleepiness scale) failed to normalize despite 3 months of CPAP treatment, even in those who were maximally compliant with treatment. Forty percent of patients in this trial had an abnormal Epworth sleepiness scale score at the conclusion of the trial128. Despite this, CPAP treatments have been shown to result in significant improvement in attention, alertness, speed of visual motion perception, vigilance, speed of information processing immediate visual memory, working memory and cognition122,126,129. A prospective 12-month observational study of CPAP treatment of OSA assessed its effects on non-motor symptoms in 67 patients with Parkinson’s disease. Overall improvement in non-motor symptoms, sleep quality, anxiety, and global cognitive function were observed130.
Diagnosis
Clinical symptoms play a key role in the diagnosis of OSA, although no sign or symptom is specific for the diagnosis of OSA. Once there is a high suspicion questionnaires and symptom- scoring scales can be used to increase the accuracy of diagnosis. Screening questionnaires are used in the outpatient setting, for symptomatic patients, to determine whether a patient should undergo polysomnography. Polysomnography is the standard for diagnostic confirmation, however it is expensive and not always available2.
The Mallampati classification (examination of the oropharyngeal inlet) is used to evaluate if tonsillar, uvular, and tongue enlargement are affecting the airway volume8.
Pathophysiology
Activation of the pro-inflammatory transcription factor nuclear kappa factor B (NF-kB) by apnea-induced hypoxia is an important pathway linking obstructive sleep apnea with systemic inflammation. It can also stimulate the downstream inflammatory markers resulting in end- organ cardiovascular disease. NF-kB activity is elevated in circulating neutrophils and monocytes in patients with obstructive sleep apnea and studies have revealed decreased activity with continuous positive airway pressure therapy in adults131-133.
Questionnaires
Questionnaires are sensitive however not very specific, therefore when a patient has low scores, it is helpful to attempt to reduce the diagnostic likelihood of OSA, in some instances avoiding the need to proceed with polysomnography134,135.
STOP-bang
The STOP-bang questionnaire includes questions on snoring, tiredness, observed apneas, blood pressure, BMI, age, neck circumference, and gender. It is one of the most sensitive questionnaires available for use in the clinical setting135. Every parameter is scored one point; and a score of >3 indicates a high risk of OSA.
Sleep apnea clinical score (SACS)
It includes data on the neck circumference, hypertension, habitual snoring, and nocturnal gasping or choking. The score ranges from zero to 100 and a score greater than 15 increases the likelihood of being positive for OSA.
Berlin questionnaire
The questionnaire has ten sections distributed in three categories, which include data on snoring, non-restorative sleep, sleepiness while driving, apneas during sleep, hypertension, and BMI. Points are assigned for each category and the patient is identified as high risk or low risk based on the points135.
NoSAS
The system assesses five components including neck circumference, BMI, snoring, age, and sex. A cut-off of eight points is used to identify patients with sleep-disordered breathing136.
Polysomnography (PSG)
PSG is the standard procedure for the diagnosis of OSA2. The preferred approach is to perform overnight PSG in the sleep laboratory; however, it is costly and may not be available (nor approved by third party insurers) at all times. Therefore, home sleep apnea testing (HSAT) can be used for certain patient populations137. HSAT can be performed for patients who have a high pre-test probability of OSA and do not have other comorbidities137. However, if a HSAT is inconclusive, inadequate or negative, PSG should be performed137. Patients with comorbidities such as cardiovascular disease, respiratory muscle weakness secondary to neuromuscular disorders, history of strokes or other ischemic disease, and chronic opioid use should undergo PSG rather than HSAT137.
Several parameters are monitored during PSG. Electroencephalography (EEG), chin electromyography (EMG) and electrooculography (EOG) are done to identify episodes of arousal and to determine sleep stage2. Respiratory airflow recommended: simultaneous monitoring of two physical variables: air temperature (for thermal airflow) and air pressure (for nasal pressure), respiratory effort, oxyhemoglobin saturation, and ECG are monitored21. To diagnose OSA, apnea-hypopnea index (AHI) is measured, i.e., the number of apneic and hypopneic episodes per hour of sleep are tabulated21. Apnea is an episode of stoppage of respiratory airflow for a minimum of 10 seconds. Hypopnea is the decrease in airflow, associated with either a drop-in oxyhemoglobin saturation or an episode of arousal21. AHI of greater than five is diagnostic of OSA. AHI greater than or equal to five but less than fifteen is classified as mild, greater than or equal to fifteen but less than thirty is classified as moderate, and greater than or equal to thirty is classified as severe OSA22.
Management of OSA
CPAP is the primary management strategy for OSA as it decreases symptoms of sleepiness and improves quality of life in patients with moderate and severe disease2,138. CPAP treatment prevents (or ameliorates) collapse of the upper airways137. Change in dietary habits, regular exercise, and weight loss can also contribute to the management of OSA139. Bariatric surgery is an option in extreme cases, and may be associated with significant improvement; however, it has not been shown to totally reverse OSA and does not replace the use of CPAP as the primary treatment140. However, after surgery and significant weight loss polysomnography should be repeated and CPAP titration should be performed141. Other surgical options include tonsillectomy, uvulopalatopharyngoplasty, tongue surgery (to reduce the size) and maxillomandibular advancement surgery142. Another alternative is the use of mandibular advancement devices (MAD). The purpose of the device is to expand and stabilize the airway and to lessen the collapse. Although these are not as effective as CPAP in reducing AHI142,143 they can be used in certain circumstances when there is insufficient compliance with CPAP use.
Wojda et al.144 performed a clinical study with 8 patients, comparing the use of CPAP and MAD and found that the symptoms improved to greater extent with CPAP. In cases where adherence to CPAP is low, these alternative options can be considered. However, CPAP is treatment of choice and has shown the best outcomes, including reduction in all-cause mortality2,138,145. Yearly follow up should be performed after CPAP is initially set up146. Oral appliances can be used for patients mild to moderate OSA under certain conditions146. Ramar et al.147 published recommendations based on an extensive review that endorsed the use of oral appliances for patients with snoring (without OSA) and for patients with OSA that are intolerant of CPAP or prefer an alternate treatment. Their guidelines also included the use of custom oral devices for patients with oversight by qualified dentists to monitor for dental side effects, and follow up testing by sleep physicians to check for treatment effectiveness.
CONCLUSION
OSA affects multiple organ systems. OSA may first present with cardiovascular or neurological morbidity, rather than respiratory symptomatology. It is important to use clinical judgement and to keep a low threshold for diagnosis when patients present with these varied signs and symptoms in order to make a timely diagnosis and to intervene to prevent morbidity and mortality.
REFERENCES
- 1.Maspero C, Giannini L, Galbiati G, Rosso G, Farronato G. Obstructive sleep apnea syndrome: a literature review. Minerva Stomatol. 2015 Apr;64(2):97–109. [PubMed] [Google Scholar]
- 2.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014 Feb;383(9918):736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 May;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickelmann AG, Burwell CS, Robin ED, Whaley RD. Extreme obesity associated with alveolar hypoventilation: a Pickwickian syndrome. Am J Med. 1956 Nov;21(5):811–818. doi: 10.1016/0002-9343(56)90094-8. [DOI] [PubMed] [Google Scholar]
- 5.Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea? The rational clinical examination systematic review. JAMA. 2013 Aug;310(7):731–741. doi: 10.1001/jama.2013.276185. [DOI] [PubMed] [Google Scholar]
- 6.Hermann DM, Bassetti CL. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology. 2016 Sep;87(13):1407–1416. doi: 10.1212/WNL.0000000000003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019 Aug;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros CA, Bruin VM, Castro-Silva C, Araújo SMHA, Chaves CM, Junior, Bruin PFC. Neck circumference, a bedside clinical feature related to mortality of acute ischemic stroke. Rev Assoc Med Bras (1992) 2011 Sep-Oct;57(5):559–564. doi: 10.1590/s0104-42302011000500015. [DOI] [PubMed] [Google Scholar]
- 9.Grunstein RR, Ho KY, Sullivan CE. Sleep apnea in acromegaly. Ann Intern Med. 1991 Oct;115(7):527–532. doi: 10.7326/0003-4819-115-7-527. [DOI] [PubMed] [Google Scholar]
- 10.Ahbab S, Ataoglu HE, Tuna M, Karasulu L, Çetin F, Temiz LU, et al. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome: evaluation of possible linkage. Med Sci Monit. 2013 Feb;19:111–117. doi: 10.12659/MSM.883776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad Med J. 2009;85(1010):693–698. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 12.McNicholas WT, Bonsigore MR, Management Committee of EU COST ACTION B26 Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007 Jan;29(1):156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 13.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005 Sep;450(6):363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 14.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006 Oct;174(7):824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 15.Lugaresi E, Cirignotta F, Coccagna G, Piana C. Some epidemiological data on snoring and cardiocirculatory disturbances. Sleep. 1980;3(3-4):221–224. doi: 10.1093/sleep/3.3-4.221. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EC, DeBehnke RD, Lovoi MS, Gorin AB. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med. 1985;103(2):190–195. doi: 10.7326/0003-4819-103-2-190. [DOI] [PubMed] [Google Scholar]
- 17.Modan M, Almog S, Fuchs Z, Chetrit A, Lusky A, Halkin H. Obesity, glucose intolerance, hyperinsulinemia, and response to antihypertensive drugs. Hypertension. 1991 Apr;17(4):565–573. doi: 10.1161/01.hyp.17.4.565. [DOI] [PubMed] [Google Scholar]
- 18.Strohl KP, Redline S. Recognition of obstructive sleep apnea. Pt 1Am J Respir Crit Care Med. 1996 Aug;154(2):279–289. doi: 10.1164/ajrccm.154.2.8756795. [DOI] [PubMed] [Google Scholar]
- 19.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997 Aug;157(15):1746–1752. [PubMed] [Google Scholar]
- 20.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javier F, Nieto, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep- disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000 Apr;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 22.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008 Feb;5(2):185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peppard PE, Young TB, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000 Dec;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 25.Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992 Feb;47(2):101–105. doi: 10.1136/thx.47.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003 Dec;42(6):1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 27.Endeshaw YW, White WB, Kutner M, Ouslander JG, Bliwise DL. Sleep-disordered breathing and 24-hour blood pressure pattern among older adults. J Gerontol A Biol Sci Med Sci. 2009 Feb;64A(2):280–285. doi: 10.1093/gerona/gln011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung RS. Sleep-disordered breathing: autonomic mechanisms and arrhythmias. Prog Cardiovasc Dis. 2009 Jan-Feb;51(4):324–338. doi: 10.1016/j.pcad.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity- associated hypertension. J Mol Med (Berl) 2001;79(1):21–29. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 30.Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013 Apr;187(8):879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 31.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012 Oct;8(5):587–596. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep- disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15(9):739–746. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulino A, Damy T, Margarit L, Stöica M, Deswarte G, Khouri L, et al. Prevalence of sleep- disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009;102(3):169–175. doi: 10.1016/j.acvd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 34.DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin- angiotensin system in the control of renal function. Hypertension. 2000 Dec;36(6):1083–1088. doi: 10.1161/01.hyp.36.6.1083. [DOI] [PubMed] [Google Scholar]
- 35.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Chiu KL, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006 Dec;174(12):1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 37.Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010 Apr;121(14):1598–1605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 38.Jordan AS, Wellman A, Edwards JK, Schory K, Dover L, MacDonald M, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol (1985) 2005 Nov;99(5):2020–2027. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dharia SM, Brown LK. Epidemiology of sleep-disordered breathing and heart failure: what drives what. Curr Heart Fail Rep. 2017 Oct;14(5):351–364. doi: 10.1007/s11897-017-0348-6. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010 Jul;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2009 Sep;14(2):131–136. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 42.Porto F, Sakamoto YS, Salles C. Association between obstructive sleep apnea and myocardial infarction: a systematic review. Arq Bras Cardiol. 2017 Apr;108(4):361–369. doi: 10.5935/abc.20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005 Mar;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 44.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007 Dec;176(12):1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 45.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016 Sep;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 46.Anandam A, Patil M, Akinnusi M, Jaoude P, El-Solh AA. Cardiovascular mortality in obstructive sleep apnoea treated with continuous positive airway pressure or oral appliance: an observational study. Respirology. 2013 Nov;18(8):1184–1190. doi: 10.1111/resp.12140. [DOI] [PubMed] [Google Scholar]
- 47.Patel N, Donahue C, Shenoy A, Patel A, El-Sherif N. Obstructive sleep apnea and arrhythmia: a systemic review. Int J Cardiol. 2017 Feb;228:967–970. doi: 10.1016/j.ijcard.2016.11.137. [DOI] [PubMed] [Google Scholar]
- 48.Youssef I, Kamran H, Yacoub M, Patel N, Goulbourne C, Kumar S, et al. Obstructive sleep apnea as a risk factor for atrial fibrillation: a meta-analysis. J Sleep Disord Ther. 2018;7(1):1000282. doi: 10.4172/2167-0277.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abumuamar AM, Dorian P, Newman D, Shapiro CM. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin Cardiol. 2018 May;41(5):601–607. doi: 10.1002/clc.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003 May;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 51.Patel D, Mohanty P, Di Biase L, Shaheen M, Lewis WR, Quan K, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010 Oct;3(5):445–451. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- 52.Monahan K, Brewster J, Wang L, Parvez B, Goyal S, Roden DM, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012 Aug;110(3):369–372. doi: 10.1016/j.amjcard.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mooe T, Gullsby S, Rabben T, Eriksson P. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypasssurgery. Coron Artery Dis. 1996 Jun;7(6):475–478. [PubMed] [Google Scholar]
- 54.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998 Jun;97(21):2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 55.Pedrosa RP, Drager LF, Genta PR, Amaro ACS, Antunes MO, Matsumoto AY, et al. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010 May;137(5):1078–1084. doi: 10.1378/chest.09-2335. [DOI] [PubMed] [Google Scholar]
- 56.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apneasyndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003 Mar;107(8):1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 57.Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012 Mar;9(3):321–327. doi: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Orban M, Bruce CJ, Pressman GS, Leinveber P, Romero-Corral A, Korinek J, et al. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation and heart failure. Am J Cardiol. 2008 Dec;102(11):1557–1561. doi: 10.1016/j.amjcard.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011 Sep;8(9):1436–1443. doi: 10.1016/j.hrthm.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 60.Shah RV, Abbasi SA, Heydari B, Farhad H, Dodson JA, Bakker JP, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014 Apr;167(4):620–626. doi: 10.1016/j.ahj.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013 Dec;2(6):e000421. doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003 May;107(20):2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 63.Bayir PT, Demirkan B, Bayir O, Duyuler S, Firat H, Güray U, et al. Impact of continuous positive airway pressure therapy on atrial electromechanical delay and P-wave dispersion in patients with obstructive sleep apnea. Ann Noninvasive Electrocardiol. 2014 May;19(3):226–233. doi: 10.1111/anec.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vural MG, Cetin S, Firat H, Akdemir R, Yeter E. Impact of continuous positive airway pressure therapy on left atrial function in patients with obstructive sleep apnoea: assessment by conventional and two-dimensional speckle-tracking echocardiography. Acta Cardiol. 2014 Apr;69(2):175–184. doi: 10.1080/ac.69.2.3017299. [DOI] [PubMed] [Google Scholar]
- 65.Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomesin patients with atrial fibrillation-results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) Am Heart J. 2015 May;169(5):647–654. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Hou Y, Po SS. Obstructive sleep apnoea and atrial fibrillation. Arrhythm Electrophysiol Rev. 2015 May;4(1):14–18. doi: 10.15420/aer.2015.4.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linz D, Hohl M, Nickel A, Mahfoud F, Wagner M, Ewen S, et al. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013 Aug;62(4):767–774. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 68.Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, Böhm M. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012 Jul;60(1):172–178. doi: 10.1161/HYPERTENSIONAHA.112.191965. [DOI] [PubMed] [Google Scholar]
- 69.Shyam S, Sushilkumar SG, Rojas-Marte G, Demir S, Saxena A, Obiagwu C, et al. Electrocardiographic associations seen with obstructive sleep apnea. Sleep Dis. 2019;2019:9704785–9704785. doi: 10.1155/2019/9704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing. Am J Respir Crit Care Med. 2006 Apr;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simantirakis EN, Schiza SI, Marketou ME, Chrysostomakis SI, Chlouverakis GI, Klapsinos NC, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airwaypressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004 Jun;25(12):1070–1076. doi: 10.1016/j.ehj.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 72.Almor JM, Flor MF, Balcells E, Cladellas M, Broquetas J, Bruguera J. Prevalence of obstructive sleep apnea syndrome in patients with sick sinus syndrome. Rev Esp Cardiol. 2006 Jan;59(1):28–32. [PubMed] [Google Scholar]
- 73.Abe H, Takahashi M, Yaegashi H, Eda S, Tsunemoto H, Kamikozawa M, et al. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apneapatients. Heart Vessels. 2010 Jan;25(1):63–69. doi: 10.1007/s00380-009-1164-z. [DOI] [PubMed] [Google Scholar]
- 74.Atwood CW, Junior, McCrory D, Garcia JG, Abman SH, Ahearn GS. Pulmonary artery hypertension and sleep-disordered breathing: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):72S–77S. doi: 10.1378/chest.126.1_suppl.72S. [DOI] [PubMed] [Google Scholar]
- 75.Frost A, Badesch D, Gibbs SR, Gopalan D, Khanna D, Manes A, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53(1):1801904–1801904. doi: 10.1183/13993003.01904-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marrone O, Bonsignore MR. Pulmonary hemodynamics in obstructive sleep apnoea. Sleep Med Rev. 2002;6:175–193. doi: 10.1053/smrv.2001.0185. [DOI] [PubMed] [Google Scholar]
- 77.Arias MA, García-Rio F, Alonso-Fernandez A, Martinez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continued positive airway pressure. Eur Heart J. 2006;27(9):1106–1113. doi: 10.1093/eurheartj/ehi807. [DOI] [PubMed] [Google Scholar]
- 78.Fagan KA. Selected contribution: pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol. 2001;95:96–100. doi: 10.1152/jappl.2001.90.6.2502. [DOI] [PubMed] [Google Scholar]
- 79.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of endothelium in pathophysiology and management. Circulation. 2000;102(14):1718–1723. doi: 10.1161/01.cir.102.14.1718. [DOI] [PubMed] [Google Scholar]
- 80.Lippi G, Mattiuzzi C, Franchini M. Sleep apnea and venous thromboembolism. A systematic review. Thromb. Haemost. 2015 Nov;114(5):958–963. doi: 10.1160/TH15-03-0188. [DOI] [PubMed] [Google Scholar]
- 81.Arzt M, Luigart R, Schum C, Luthje L, Stein A, Koper I, et al. Sleep-disordered breathing in deep vein thrombosis and acute pulmonary embolism. Eur Respir Rev. 2012 Oct;40(4):919–924. doi: 10.1183/09031936.00176711. [DOI] [PubMed] [Google Scholar]
- 82.Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008 Mar;358(10):1037–1052. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 83.Ambrosetti M, Lucioni A, Ageno W, Conti S, Neri M. Is venous thromboembolism more frequent in patients with obstructive sleep apnea syndrome? J Thromb Harmost. 2004;2(10):1858–1850. doi: 10.1111/j.1538-7836.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- 84.Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med. 2002;162(11):1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 85.Tsai JCG. Neurological and neurobehavioral sequelae of obstructive sleep apnea. Neuro Rehabilitation. 2010;26(1):85–94. doi: 10.3233/NRE-2010-0538. [DOI] [PubMed] [Google Scholar]
- 86.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008 Aug;118(9):955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 87.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005 Nov;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 88.Dong R, Dong Z, Liu H, Shi F, Du J. Prevalence, risk factors, outcomes, and treatment of obstructive sleep apnea in patients with cerebrovascular disease: a systematic review. J Stroke Cerebrovasc Dis. 2018 Jun;27(6):1471–1480. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 89.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006 Apr;37(4):967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 90.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005 Mar;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 91.Iranzo A, Santamaría J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002 Mar;58(6):911–916. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 92.Kim JS, Kim S, Lee SH, Lee HY, Lee SY, Im KB. Increased risk of ischemic stroke during sleep in apneic patients. J Clin Neurol. 2018 Apr;14(2):174–178. doi: 10.3988/jcn.2018.14.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alvarez-Sabín J, Romero O, Delgado P, Quintana M, Santamarina E, Ferré A, et al. Obstructive sleep apnea and silent cerebral infarction in hypertensive individuals. J Sleep Res. 2018 Apr;27(2):232–239. doi: 10.1111/jsr.12571. [DOI] [PubMed] [Google Scholar]
- 94.Bålfors EM, Franklin KA. Impairment of cerebral perfusion during obstructive sleep apneas. Pt 1Am J Respir Crit Care Med. 1994 Dec;150(6):1587–1591. doi: 10.1164/ajrccm.150.6.7952619. [DOI] [PubMed] [Google Scholar]
- 95.Durgan DJ, Bryan RM. Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc. 2012 Aug;1(4):e000091. doi: 10.1161/JAHA.111.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Urbano F, Roux F, Schindler J, Mohsenin V. Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol (1985) 2008 Dec;105(6):1852–1857. doi: 10.1152/japplphysiol.90900.2008. [DOI] [PubMed] [Google Scholar]
- 97.Nasr N, Traon AP, Czosnyka M, Tiberge M, Schmidt E, Larrue V. Cerebral autoregulation in patients with obstructive sleep apnea syndrome during wakefulness. Eur J Neurol. 2009;16:386–391. doi: 10.1111/j.1468-1331.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- 98.Ponsaing LB, Lindberg U, Rostrup E, Iversen HK, Larsson HBW, Jennum P. Impaired cerebrovascular reactivity in obstructive sleep apnea: a case-control study. Sleep Med. 2018 Mar;43:7–13. doi: 10.1016/j.sleep.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 99.Jensen MLF, Vestergaard MB, Tønnesen P, Larsson HBW, Jennum PJ. Cerebral blood flow, oxygen metabolism, and lactate during hypoxia in patients with obstructive sleep apnea. Sleep. 2018 Mar;41(3):zsy001. doi: 10.1093/sleep/zsy001. [DOI] [PubMed] [Google Scholar]
- 100.Diomedi M, Placidi F, Cupini LM, Bernardi G, Silvestrini M. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology. 1998 Oct;51(4):1051–1056. doi: 10.1212/wnl.51.4.1051. [DOI] [PubMed] [Google Scholar]
- 101.Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Effects of continuous positive airway pressure on cerebral vascular response to hypoxia in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2007 Apr;175(7):720–725. doi: 10.1164/rccm.200609-1271OC. [DOI] [PubMed] [Google Scholar]
- 102.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26(3):293–297. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 103.Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax. 2004 May;59(5):367–371. doi: 10.1136/thx.2003.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar R, Suri JC, Manocha R. Study of association of severity of sleep disordered breathing and functional outcome in stroke patients. Sleep Med. 2017 Jun;34:50–56. doi: 10.1016/j.sleep.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 105.Scala R, Turkington PM, Wanklyn P, Bamford J, Elliott MW. Acceptance, effectiveness and safety of continuous positive airway pressure in acute stroke: a pilot study. Respir Med. 2009;103:59–66. doi: 10.1016/j.rmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J. 2017 Jun;39(24):2291–2297. doi: 10.1093/eurheartj/ehx597. [DOI] [PubMed] [Google Scholar]
- 107.Tsivgoulis G, Alexandrov AV, Katsanos AH, Barlinn K, Mikulik R, Lambadiari V, et al. Noninvasive ventilatory correction in patients with acute ischemic stroke: a systematic review and meta-analysis. Stroke. 2017 Aug;48(8):2285–2288. doi: 10.1161/STROKEAHA.117.017661. [DOI] [PubMed] [Google Scholar]
- 108.Stevens D, Martins RT, Mukherjee S, Vakulin A. Post-stroke sleep-disordered breathing- pathophysiology and therapy options. Front Surg. 2018 Feb;5:9–9. doi: 10.3389/fsurg.2018.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun MH, Liao YJ, Lin CC, Chiang RP, Wei JC. Association between obstructive sleep apnea and optic neuropathy: a Taiwanese population-based cohort study. Eye (Lond) 2018 Aug;32(8):1353–1358. doi: 10.1038/s41433-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaitanya A, Pai VH, Mohapatra AK, Ve RS. Glaucoma and its association with obstructive sleep apnea: A narrative review. Oman J Ophthalmol. 2016 Sep-Dec;9(3):125–134. doi: 10.4103/0974-620X.192261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsumura E, Matas CG, Sanches SGG, Magliaro FCL, Pedreño RM, Genta PR, et al. Severe obstructive sleep apnea is associated with cochlear function impairment. Sleep Breath. 2018 Mar;22(1):71–77. doi: 10.1007/s11325-017-1530-5. [DOI] [PubMed] [Google Scholar]
- 112.Zhou L, Ouyang R, Chen P, Luo H, Liu H, Liu G. A case of severe obstructive sleep apnea hypopnea syndrome with urinary and anal incontinence. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018 Mar;43(3):333–336. doi: 10.11817/j.issn.1672-7347.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 113.McInnis RP, Dodds EB, Johnsen J, Auerbach S, Pyatkevich Y. CPAP treats enuresis in adults with obstructive sleep apnea. J Clin Sleep Med. 2017 Oct;13(10):1209–1212. doi: 10.5664/jcsm.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen JH, Huang R, Luo JM, Xiao Y, Zhang Y. Adult monosymptomatic nocturnal enuresis with obstructive sleep apnea syndrome. Chin Med J (Engl) 2016 Aug;129(16):2011–2012. doi: 10.4103/0366-6999.187853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001 Jun;163(7):1626–1631. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- 116.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002 Mar;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 117.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003 May;26(3):298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 118.Décary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000 May;23(3):369–381. [PubMed] [Google Scholar]
- 119.Rouleau I, Decary A, Chicoine AJ, Montplaisir J. Procedural skill learning in obstructive sleep apnea syndrome. Sleep. 2002 Jun;25(4):401–411. [PubMed] [Google Scholar]
- 120.Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2002;24(1):93–100. doi: 10.1076/jcen.24.1.93.973. [DOI] [PubMed] [Google Scholar]
- 121.Verstraeten E, Cluydts R, Pevernagie D, Hoffmann G. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep. 2004 Jun;27(4):685–693. [PubMed] [Google Scholar]
- 122.Alchanatis M, Zias N, Deligiorgis N, Amfilochiou A, Dionellis G, Orphanidou D. Sleep apnea- related cognitive deficits and intelligence: an implication of cognitive reserve theory. J Sleep Res. 2005 Mar;14(1):69–75. doi: 10.1111/j.1365-2869.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 123.Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003 Jun;61(1):87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 124.Kingshott RN, Vennelle M, Hoy CJ, Engleman HM, Deary IJ, Douglas NJ. Predictors of improvements in daytime function outcomes with CPAP therapy. Am J Respir Crit Care Med. 2000;161(3):866–871. doi: 10.1164/ajrccm.161.3.9905053. [DOI] [PubMed] [Google Scholar]
- 125.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999 Jun;353(9170):2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 126.Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo- controlled trial. Am J Respir Crit Care Med. 2001;163(4):911–917. doi: 10.1164/ajrccm.163.4.9910025. [DOI] [PubMed] [Google Scholar]
- 127.Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998 May;53(5):341–345. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Antic NA, Catcheside P, Buchan C, Hensley M, Naughton MT, Rowland S, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011 Jan;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aaronson JA, Hofman WF, van Bennekom CA, van Bezeij T, van den Aardweg JG, Groet E, et al. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2016 Apr;12(4):533–541. doi: 10.5664/jcsm.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaminska M, Mery VP, Lafontaine AL, Robinson A, Benedetti A, Gros P, et al. Change in cognition and other non-motor symptoms with obstructive sleep apnea treatment in Parkinson disease. J Clin Sleep Med. 2018 May;14(5):819–828. doi: 10.5664/jcsm.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005 Oct;112(17):2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 132.Htoo AK, Greenberg H, Tongia S, Chen G, Henderson T, Wilson D, et al. Activation of nuclear factor kappa B in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006 Mar;10(1):43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 133.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappa B in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006 May;343(2):591–596. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 134.Amra B, Javani M, Soltaninejad F, Penzel T, Fietze I, Schoebel C, et al. Comparison of Berlin Questionnaire, STOP-Bang, and Epworth Sleepiness Scale for Diagnosing Obstructive Sleep Apnea in Persian Patients. Int J Prev Med. 2018 Mar;9(1):28–28. doi: 10.4103/ijpvm.IJPVM_131_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiu HY, Chen PY, Chuang LP, Chen NH, Tu YK, Hsieh YJ, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017 Dec;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 136.Marti-Soler H, Hirotsu C, Marques-Vidal P, Vollenweider P, Waeber G, Preisig M, et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016 Sep;4(9):742–748. doi: 10.1016/S2213-2600(16)30075-3. [DOI] [PubMed] [Google Scholar]
- 137.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017 Mar;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006 Jan;(1):CD001106. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 139.Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010 Oct;24(5):703–715. doi: 10.1016/j.beem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dixon JB, Schachter LM, O'Brien PE, Jones K, Grima M, Lambert G, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012 Sep;308(11):1142–1149. doi: 10.1001/2012.jama.11580. [DOI] [PubMed] [Google Scholar]
- 141.Priyadarshini P, Singh VP, Aggarwal S, Garg H, Sinha S, Guleria R. Impact of bariatric surgery on obstructive sleep apnoea-hypopnea syndrome in morbidly obese patients. J Minim Access Surg. 2017 Oct-Dec;13(4):291–295. doi: 10.4103/jmas.JMAS_5_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sarkissian L, Kitipornchai L, Cistulli P, Mackay SG. An update on the current management of adult obstructive sleep apnoea. Aust J Gen Pract. 2019 Apr;48(4):182–186. doi: 10.31128/AJGP-10-18-4749. [DOI] [PubMed] [Google Scholar]
- 143.Cammaroto G, Galletti C, Galletti F, Galletti B, Galletti Cl, Gay-Escoda C. Mandibular advancement devices vs nasal-continuous positive airway pressure in the treatment of obstructive sleep apnoea. Systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2017 Jul;22(4):e417–e424. doi: 10.4317/medoral.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wojda M, Kostrzewa-Janicka J, Sliwinski P, Bielen P, Jurkowski P, Wojda R, et al. Mandibular advancement devices in obstructive sleep apnea patients intolerant to continuous positive airway pressure treatment. Adv Exp Med Biol. 2019;1150:35–42. doi: 10.1007/5584_2018_275. [DOI] [PubMed] [Google Scholar]
- 145.Lisan Q, van Sloten T, Vidal PM, Rubio JH, Heinzer R, Empana JP. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the Sleep Heart Health Study. JAMA Otolaryngol Head Neck Surg. 2019 Jun;145(6):509–515. doi: 10.1001/jamaoto.2019.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Epstein LJ, Kristo D, Strollo PJ, Junior, Friedman NN, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009 Jun;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 147.Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, et al. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. An American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2015 Jul;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]