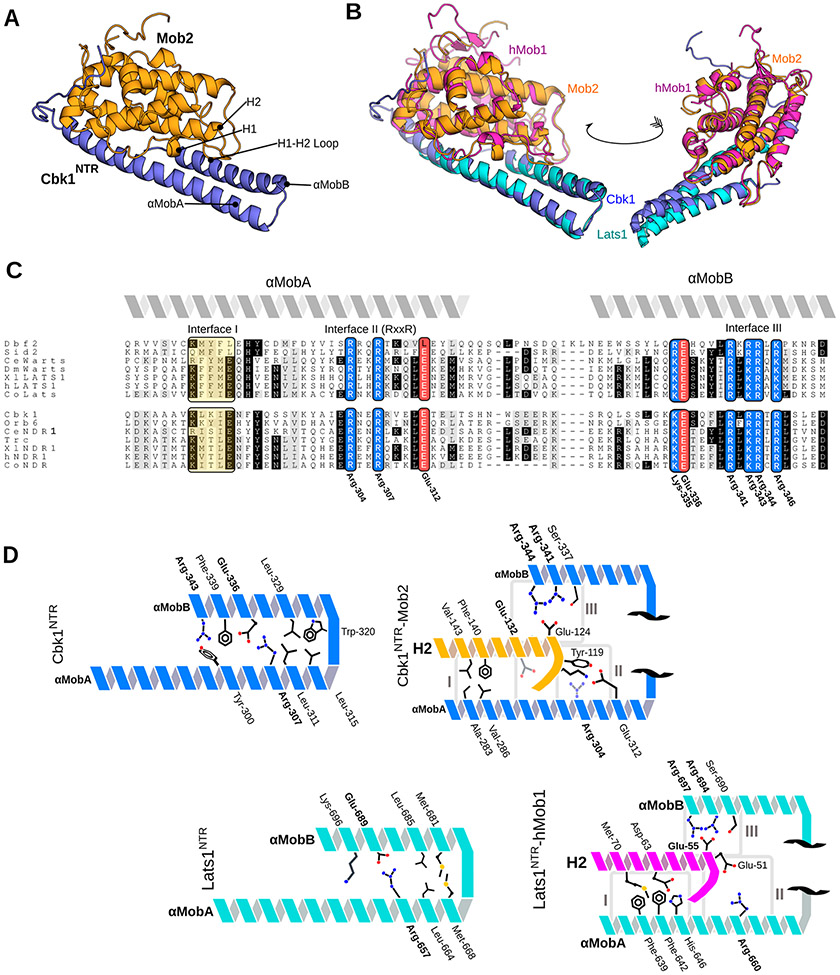
Figure 1.
Crystal structure of the Cbk1NTR–Mob2 complex that highlights conserved structural motifs at the Ndr/Lats–Mob interface. (A) Cbk1NTR (residues 251–351, blue) complexed with zinc-binding Mob2 (residues 45–287, orange). (B) Cbk1NTR–Mob2 (blue/orange) overlaid with Lats1NTR–hMob1 (PDB entry 5B5W, cyan/purple). (C) Alignment of the Ndr/Lats NTR regions across eukaryotes. Abbreviations: Ce, Caenorhabditis elegans; Dm/Wts, Drosophila melanogaster; Xl, Xenopus laevis; Co, Capsaspora owczarzaki. Basic residues are colored blue, acidic residues red, and hydrophobic regions yellow. Interfaces I–III equivalent to those described in the Lats1–hMob1 structure are labeled. Conserved residues shown in Cbk1 are labeled. (D) Cbk1NTR–Mob2 (top) and Lats1NTR–Mob1 interaction architecture. The NTR is comprised of two Mob-binding helices: αMobA and αMobB. Conserved residues are labeled in bold.