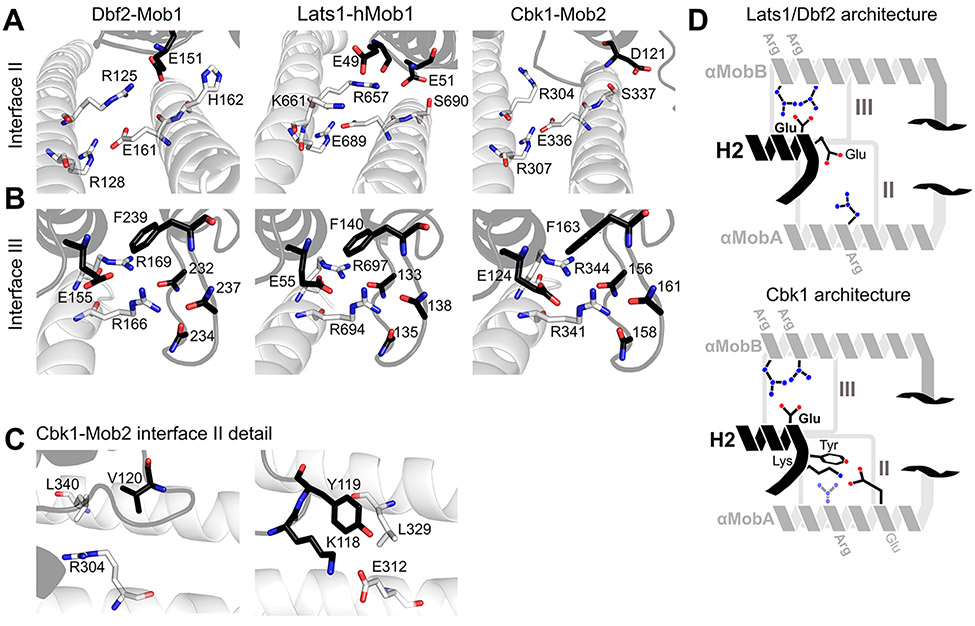
Figure 3.
Conserved regions cohere Ndr/LatsNTR–Mob complexes. (A) Comparison of interface II from Lats1NTR–hMob1 (PDB entry 5B5W, center panel) with Cbk1NTR–Mob2 (right) and Dbf2NTR–Mob1 (left). Highlighted residues shown as sticks are important for the interaction. (B) Comparisons of interface III from Lats1NTR–hMob1 (center), Cbk1NTR–Mob2 (right), and Dbf2NTR–Mob1 (left). (C) More detailed view of interface II of the Cbk1NTR–Mob2 complex showing the orientation of Cbk1 Arg-304 in relation to Leu-340 and Mob2 Val-120 (left) and Cbk1 Glu-312 and Mob2 Lys-118 and Tyr-119 (right). The interaction between Lys-118/Tyr-119 of Mob2 and Glu-312/Leu-329 of Cbk1 is distinct from those of Lats and Dbf2–Mob1 complexes. (D) Generalized architecture of Dbf2/Lats1–Mob1 in relation to that of Cbk1–Mob2. Conserved binding interfaces II and III as shown in panels A–C are boxed. Basic residues are colored blue, and acidic residues red. Mob is colored black, and the Ndr/Lats kinase NTR is colored gray.