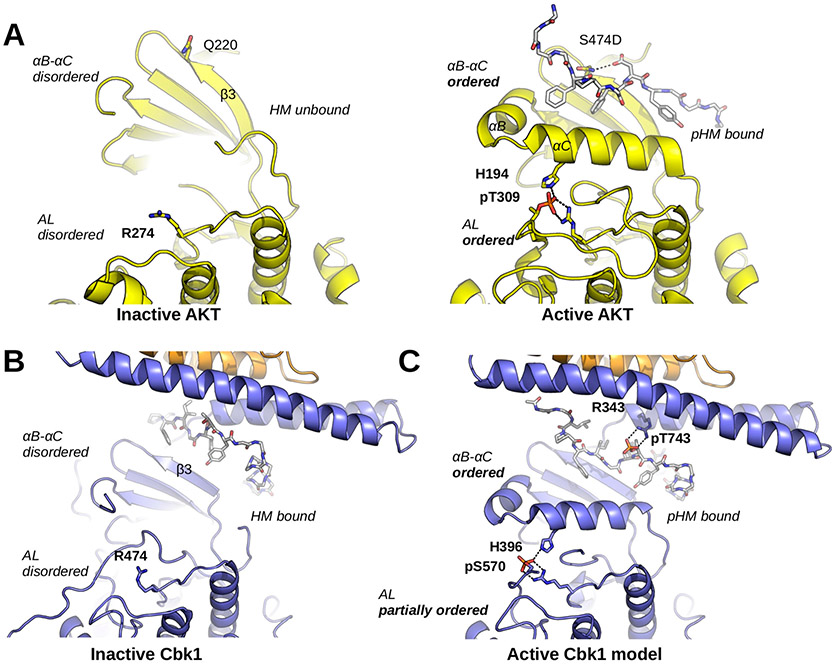
Figure 6.
Cbk1 activation: the role of HM phosphorylation and Mob binding. (A) The activation of AKT/PKB involves a disorder-to-order transition at the αB–αC segment, the activation loop (AL), and the HM. Q220 coordinates the phosphorylated HM (here shown with the S474D phosphomimicking mutation; PDB entry 1O6K). The phosphorylated activation loop (AL; phosphoThr-309) is coordinated by Arg-274 and His-194 (from αC). (B) The inactive Cbk1 structure shows a similar structure apart from the position of its HM. The inactive crystal structure of the Cbk1–Mob2 complex shows that the HM is bound to the groove formed by the Mob-bound NTR and β3 from Cbk1. (C) For activation, the ordering of helix αC in Cbk1 similar to that of AKT/PKB is likely required (MD model). This latter may be supported by interactions formed by phospho-HM and phospho-AL. The activation loop phosphosite (AKT/PKB, Thr-309; Cbk1, Ser-570) is coordinated by an arginine residue from the kinase HRD motif (AKT/PKB, Arg-274; Cbk1, Arg-474) and by another residue from helix αC (AKT/PKB, His-194; Cbk1, His-396). The hydrophobic motif phosphosite (AKT/PKB, Ser-474; Cbk1, Thr-743) in most AGC kinases is coordinated by a single residue from β3 (AKT/PKB, Gln-220). In the case of Cbk1, an arginine residue (Arg-343) from the Mob-bound αMobB coordinates the HM phosphosite while β3 may not be involved. For both AKT/PKB and Cbk1, phospho-HM coordination (by Gln-220-pSer-474 or by Arg-343-pThr-743) brings hydrophobic residues much closer to αC and probably facilitates its ordering. This activation model suggests that coactivator binding is essential not only in HM binding but also in the precise coordination of the kinase’s active state when it is phosphorylated. AKT/PKB is colored yellow, and both HM regions are shown with gray sticks.