FIGURE 11.
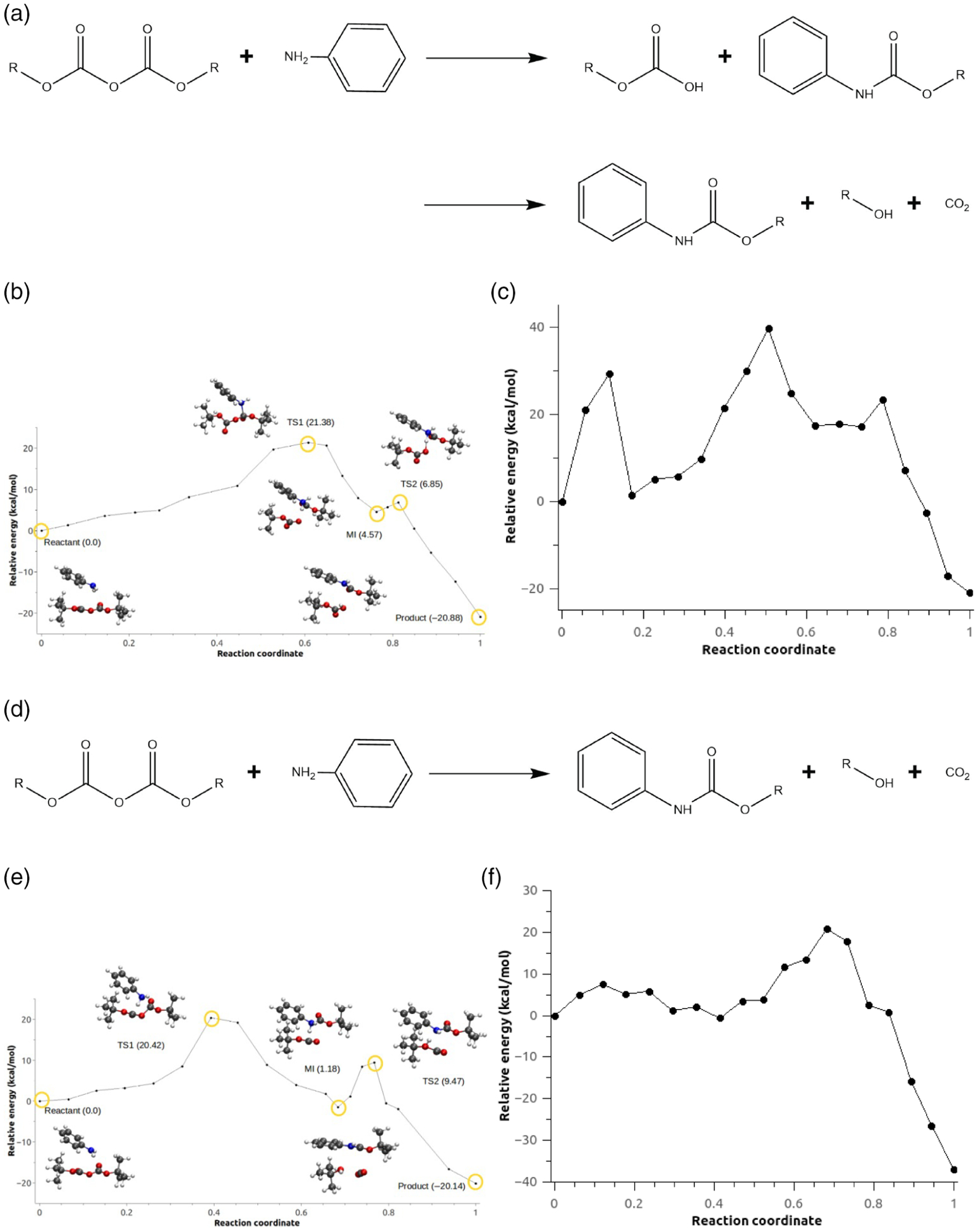
(a) Reaction scheme for the N-tert-butoxycarbonylation of aniline for the step-wise mechanism. (b) Minimum energy path for configuration C2, Scheme 1c. (c) Minimum energy path for Scheme c for configuration C2 without the AMOEBA polarization term. (d) Reaction scheme for the N-tert-butoxycarbonylation of aniline for the concerted mechanism. (e) Minimum energy path for configuration C2, Scheme 1d. (f) Minimum energy path for Scheme d for configuration C2 without the AMOEBA polarization term
Source: Adapted from E.A. Vazquez-Montelongo, J.E. Vazquez-Cervantes, G.A. Cisneros, Polarizable ab initio QM/MM study of the reaction mechanism of N-tert-butyloxycarbonylation of aniline in [EMIm][BF4]. Molecules 2018, 23, 283025
