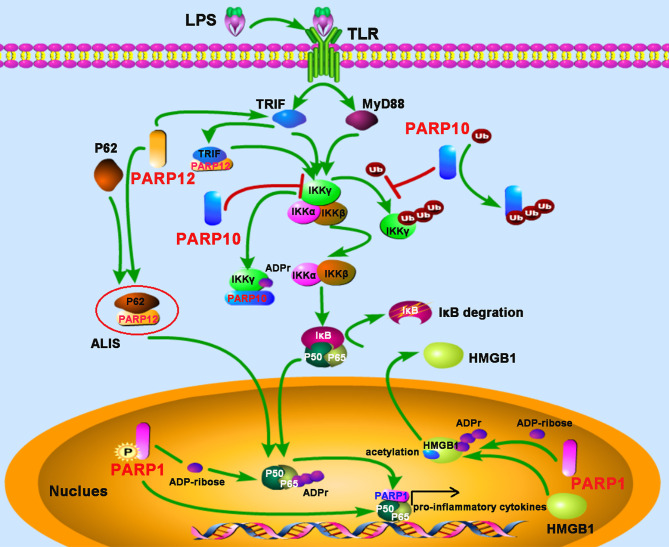
Figure 3.
PARPs regulate the NF-κB-mediated inflammatory responses. LPS stimulates the activation of the NF-κB signaling pathway depending on TRIF (toll-receptor-associated activator of interferon) and MyD88 (myeloid differentiation factor 88). PARP12 is recruited and co-localized with p62 in the aggresome-like induced structures (ALIS), which acts as a platform for NF-κB signaling; PARP12 interacts and co-localizes with TRIF, leading to an enhancement of NF-κB signaling. PARP10 inhibits the activation of IKKγ through two mechanisms: competitively binding of K63-polyubiquitination to interfere with IKKγ’s polyubiquitylation and interacting with IKKγ to promote its mono-ADP-ribosylation, leading to the inhibition of p65 translocation into the nucleus. The phosphorylation of PARP1 results in the PARylation of the NF-κB subunit p65. The direct interaction with both subunits of NF-κB (p50 and p65) by PARP1 promotes NF-κB-inducible cytokine production. PARP1 mediates the PARylation of high-mobility group box 1 (HMGB1), following by subsequent acetylation, and then induces its release from the nucleus to the cytoplasm.