Figure 1.
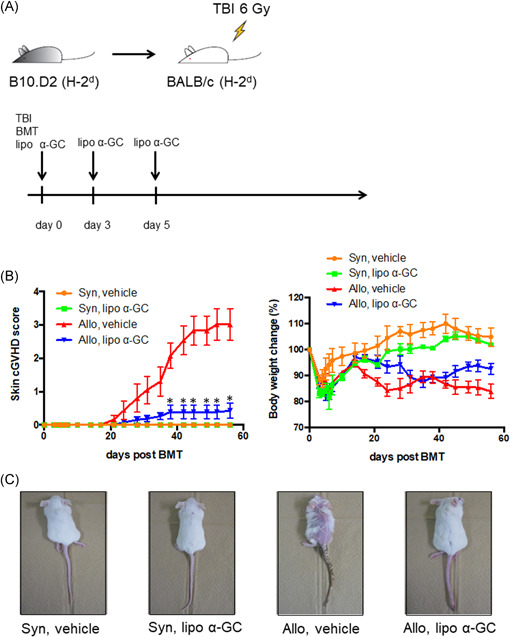
Lipo α‐GC prevents cGVHD in a sclerodermatous cGVHD mouse model. (A) Experimental scheme. BALB/c recipient mice received 6 Gy of TBI; then, 8 × 106 BM cells and 10 × 106 spleen cells were administered from B10.D2 donor mice. Lipo α‐GC was administered at a dose of 1 μg/kg via tail vein injection on Days 0, 3, and 5 after BMT. (B) Time courses of skin cGVHD score and percent body weight changes from baseline after BMT in syngeneic/vehicle (n = 3), syngeneic/lipo α‐GC (n = 3), allogeneic/vehicle (n = 8), and allogeneic/lipo α‐GC (n = 8) groups. Representative data from one of three independently performed experiments are expressed as the means ± SEM. p‐values were determined by the Kruskal–Wallis test; *p < .05. (C) Representative photographs of mice from syngeneic/vehicle (n = 3), syngeneic/lipo α‐GC (n = 3), allogeneic/vehicle (n = 8), and allogeneic/lipo α‐GC (n = 8) groups on Day 56 after BMT from one of three independently performed experiments. BMT, bone marrow transplantation; BM, bone marrow; cGVHD, chronic graft‐versus‐host disease; α‐GC, α‐Galactosylceramide
