Abstract
Globally, textile industries are one of the major sectors releasing dye pollutants. This is the first report on the positive correlation between toxicity and chemical oxygen demand (COD) of textile effluent along with the proposed pathway for enzymatic degradation of acid orange 10 using Geotrichum candidum within a very short stretch of time (18 h). Removal efficiency of this mycoremedial approach after 18 h in terms of chemical oxygen demand, biological oxygen demand, total suspended solids, salinity, color and dye concentration in the treated effluent reached to 98.5%, 56.3%,73.2%, 64%, 89% and 87% respectively. Also there was a decrease in pH of the treated effluent. FTIR analysis of the treated effluent confirmed biodegradation. The LCMS analysis showed the degradation of acid orange 10, which was confirmed by the formation of two biodegradation products, 7-oxo-8-iminonapthalene-1,3-disulfonate and nitrosobenzene, which subsequently undergoes stepwise hydrogenation and dehydration to form aniline via phenyl hydroxyl amine as intermediate. The X-ray diffraction studies showed that heavy metal content in the treated effluent has reduced along with decrease in % crystallinity, indicating biodegradation. The connection between toxicity and COD was also inveterated using Pearson’s correlation coefficient. Further the toxicological studies indicated the toxicity of raw textile effluent and relatively lower toxic nature of metabolites generated after biodegradation by G. candidum.
Subject terms: Biological techniques, Biotechnology, Microbiology, Environmental sciences
Introduction
Urbanization and industrialization have paved the path for development of the many industries, including the textile industries. Clothing and textiles, after agriculture, is the basic requirement of human being. While the textile industry contributes worldwide economically, the environmental effects are due to high volumes of water use and the diversity and quantities of chemicals that are used in all manufacturing phases of textiles. The untreated effluent when disposed in the water bodies seriously impacted the people in the area1. Rivers and drainage bodies get loaded with precarious textile effluents that impact on the water quality, aquatic organisms and human life2–7. The diverse sort of dyes and chemicals used in textile manufacturing makes textile effluents very complex in terms of chemical compositions. According to previous records, in addition to dyes and its auxiliaries over 8000 chemicals are added such as several acids, salts, surfactants, metals, oxidizing and reducing agents8. These recalcitrants in untreated effluents are both harmful to marine and terrestrial organisms and have prolonged effects on health9.
To assess the performance of wastewater treatment facilities, the influent and treated water samples after each treatment phase (physical, chemical and biological) should be tracked. In general, microbial degradation or bioremediation is known to be a safe, natural, inexpensive and effective pollutant removal technique in the world7,10–12. Bioremediation is an innovative clean-up technology that involves the use of bacteria, fungi, actinomycetes, and earthworms13. Because of its low cost, high efficiency, and eco-friendly nature, it is a sustainable process for the treatment of organics-rich solid wastes and wastewater produced from various sources14. Bioremediation appears to be a promising alternative to other widely used clean-up technologies, such as photocatalytic degradation, because studies have shown that the intermediates released during the photocatalytic degradation process are detrimental to a variety of organisms in the environment15–18. Moreover, these processes are also associated with high-energy consumption and capital cost.
However, bacteria typically contribute to the breakdown of textile dyes creating and accumulating more intractable or hazardous aromatic amine substances that restrict their comprehensive applications to azo dye wastewater treatment plants19. Enhanced techniques are evidently the pre-condition for accelerated elimination of azo dyes, because any residual contaminants should be removed completely. Fungi have been investigated, especially those secreting non-specific oxidases which eventually lead to the azo dyes mineralization into CO220. Fungal degradation (Mycoremediation) also leads to complete discoloration and detoxification, which prevents sludge removal and secondary contamination issues.
Geotrichum sp. is one of the few fungi found to degrade large amounts of artificial colors and molasses21–26. Because Geotrichum sp has received little attention, it is being used in the current study for the biodegradation and detoxification of textile effluent. In order to confirm the degradation efficiency of the fungus, toxicity evaluation is a requisite. The studies based on toxicity assessments could be done with bioassays using all forms of harmful compounds found in textile effluent and may well be utilized to evaluate the effect of unidentified compounds which cause detrimental, additive and synergistic effects27,28.
In the current study, an attempt to validate the non-toxicity of mycoremediated textile effluent as well as its biodegradation analysis such as FTIR, LCMS and XRD have been carried out. Furthermore, bioassays such as genotoxicity, phytotoxicity and microbial toxicity assays have also been conducted so that the toxicity level of raw and treated textile effluent could be assessed.
Materials and methods
Collection of samples
Samples of textile dyeing effluent have been obtained from a nearby textile factory in Khurda, India. During regular operations, factory workers sampled 100 mL of wastewater every two hours to ensure that the study involves variability in substances.
Microbial culture conditions
This work utilizes Geotrichum candidum, a ubiquitous fungus belonging to Dipodoascaceae family. It was grown at 35 °C on Potato Dextrose Agar plates (PDA) (pH-5.6 ± 0.2). Pure fungal culture was inoculated in 3% malt extract broth after 24 h to maintain the strain and cultured at 35 °C, 100 rpm. Using DP media (dextrose and peptone in ratio of 2:3) at 35 °C and 100 rpm, the optimum fungal growth was achieved25.
Analysis of conventional indicators of textile effluent
In this part of the analysis, the G. candidum culture was used to biodegrade the textile effluent. A conical flask containing raw textile effluent (25 ml) was inoculated with fungal culture (5%, v/v), followed by incubation at 35 °C, 100 rpm25. At regular intervals, aliquots were obtained from the flask and then centrifuged (10,000 × g) for 10 min. Thereafter, the conventional indicators for raw and treated effluent such as Chemical Oxygen Demand (COD), Biological Oxygen Demand (BOD), Total Suspended Solids (TSS), pH, salinity, color and concentration were assessed following the Standard Methods29. COD concentrations were measured using the potassium dichromate method; Pt–Co color scale was used to measure color. TSS estimation was carried out by simple laboratory method30. The pH of water was determined by using a glass electrode pH meter (Systronics India, Model 362) and salinity was measured by Hanna Salinity Tester (HI98319). Dye concentration of the sample was determined using colorimeter (Systonic, S-912). Every experiment has been carried out in triplicates and standard deviation has been presented with the average data.
The following equation (Eq. (1)) was used to quantify degradation as a percentage reduction of COD:
| 1 |
COD initial: initial value of COD. COD t: value of COD at time ‘t’ (h).
Growth kinetics
The following Eq. (2) has been used to determine the specific growth rate of G. candidum.
| 2 |
x: biomass concentration (g L−1) at ‘t’ time. xo: initial biomass concentration (g L−1) at ‘t0’ time. μ: specific growth rate (h–1).
The expression for growth yield (Y) is
| 3 |
The Eq. 3 can be rewritten as follows 31:
| 4 |
So: initial substrate (COD) concentration (mg L−1). S: final substrate (COD) concentration (mg L−1). x: biomass concentration (mg L–1). xo: initial biomass concentration (mg L−1).
Analytical studies
The metabolites formed in textile effluent after decolorization and degradation were obtained by same volume with ethyl acetate. The extract was dried over anhydrous sodium sulfate and evaporated to dryness in a rotary evaporator. The resulting crystals were dissolved in small volumes of methanol (HPLC grade), and then used for analysis such as FTIR (Fourier-transform infrared spectroscopy), LCMS (Liquid chromatography–mass spectrometry) and XRD (X-ray Diffraction).
The FTIR analysis of the effluent was performed with Attenuated total reflectance- Fourier transform infrared spectroscopy (ATR-FTIR, FT/IR-4600, JASCO, Japan). A drop from each sample was placed on a Zinc selenide (ZnSe) frame, and the spectra were documented with an average of 32 scans between the 4000 and 600 cm−1 spectral ranges.
The raw and treated samples were also analyzed using LCMS (Waters Micromass Q-Tof Micro) and the flow rate and temperature were maintained at 0.2 ml min−1 and 35 °C, respectively. The running time was 41 min. Two different solvents with varying proportions, such as water with 0.1% formic acid and acetonitrile with 0.1% formic acid were used. The deuterium lamp (DL) temperature was set at 250 °C with m/z value 50–1000 runs in the positive ion mode.
X-ray diffraction patterns before and after biodegradation of textile effluent were recorded using RIGAKU-ULTIMA IV, Japan diffractometer with monochromatic CuKα radiation (λ = 1.5406) over the range of 10–90° (2θ). The metals were identified with powder diffraction standard file (JCPDS, Joint Committee on Powder Diffraction Standards Newtown Square, Pennsylvania, USA).
Toxicity study
Phytotoxicity
The phytotoxicity analysis was performed using Phaseolus mungo (at room temperature) on both raw and treated effluent. Simultaneously, the control set was conducted using tap water. After 7 days, toxicity of the raw and treated effluent was evaluated by the length of radical, plumule and germination percentage. Mean with standard deviation for all results were presented.
Microbial toxicity
A short-term toxicity test of textile effluent before and after treatment was demonstrated by exposing the bacteria Escherichia coli (ATCC 443) to the textile effluent for 15 min. Toxicity to E. coli was determined spectrophotometrically by evaluating the difference in the number of cells before and after treatment. All the tests were conducted in triplicate. An experiment with a control set was also carried out.
Genotoxicity Study
The study of genotoxicity was carried out with Allium cepa. Both raw and treated textile effluent was used to treat the roots. The growths after 48 h of incubation at room temperature were examined32. A light microscope (NikonH600L Eclipses LV100) was used to obtain mean values of root length, mitotic index (MI) and chromosomal aberrations in the cell. The experiment was conducted in triplicates and the mean ± standard deviation values were accounted.
Statistical analysis
The toxicity test results for E. coli were expressed as EC50, which represented a 50% inhibition of E. coli growth caused by a percentage concentration of the textile effluent (v/v). EC50 was evaluated via the linear interpolation method33.
The Linear Regression method is to fit concentration– inhibition data to a linear regression and then to calculate EC50 values by linear interpolation. The Interpolation log method is according to Huber and Koella34 using the following formula, Eq. (5) to calculate the EC50 value.
| 5 |
Direct Interpolation method is similar to interpolation log method but without logarithmic transformation of concentrations. The equation Eq. (6) for EC50 calculation is according to 35 as follows.
| 6 |
x1, x2: two conc. of the textile effluent; y1, y2: corresponding inhibitions (y1 < 50%; y2 > 50%). % Inhibition can be calculated from the following formula (7)36
| 7 |
After evaluation of EC50, the toxicity of all samples were expressed as toxic units, TU (unitless), as per Eq. (8) 37,
| 8 |
The Pearson’s correlation coefficient at the significance level of 0.05 was used to assess the correlation between toxicity and conventional indicators of the raw and treated textile effluent, and the impact of COD on toxicity was analyzed by means of linear regression. The significance level of the regression analysis (p) and R2 illustrated the extent of toxicity variance caused by COD.
Results and discussion
Conventional indicators of textile effluent
The conventional indicators of raw and treated textile effluent have been listed in Table 1. It was evident that the raw textile effluent was high in COD, BOD, TSS and salinity38, which was way much higher than the permitted levels (COD- less than 250 mg L−1, BOD- less than 30 mg L−1, TSS- less than 100 mg L−1 in India). Studies show that common components of dye effluents such as some acid dyes and ionic dyes could easily escape into the environment leading to elevating levels of COD, BOD, TSS, salinity and coloration of water bodies39. After treatment, it was observed that G. candidum was able to remove COD (98.5%), BOD (56.3%), TSS (73.2%), salinity (64%), dye concentration (87%), color (89%) from the textile effluent in 18 h, which meets the discharge standards. Also the pH of the treated effluent was found to drop and reach neutral point. The high removal efficiency of G. candidum improved the quality of textile effluent in terms of COD, BOD and color. A recent study showed the reduction in COD, BOD and color of the textile effluent by 77.5%, 71.0% and 99.2% respectively in 24 h after treatment with Aspergillus niger40. Yet another study showed that 91%, 88% and 68% reduction was recorded in the color intensity, COD and BOD of the textile wastewater, respectively, after treatment with Peyronellaea prosopidis41.
Table 1.
Conventional indicators in raw and treated textile effluent.
| Indicators | Raw textile effluent | Treated textile effluent (after 18 h) | Removal (%) (after 18 h) |
|---|---|---|---|
| COD (mg L−1) | 902.1 ± 0.05 | 13.20 ± 0.05 | 98.5 |
| BOD (mg L−1) | 350.3 ± 0.02 | 153.08 ± 0.02 | 56.3 |
| pH | 7.9 | 7 | - |
| TSS (mg L−1) | 320 ± 0.05 | 85.5 ± 0.05 | 73.2 |
| Salinity (PSU)* | 0.25 | 0.09 | 64 |
| Color (hazen units) | 35,400 | 3894 | 89 |
| Concentration of sample(ppm) | 3100 | 400 | 87 |
*PSU- Practical Salinity Units.
Growth kinetics studies
The specific growth rate of 0.127 h–1 and yield coefficient of 2.64 mg of dry weight of biomass mg−1 COD were obtained for the biodegradation using G. candidum. This indicated that G. candidum was able to thrive in the extreme conditions of the effluent, reducing COD efficiently. The literatures reported the specific growth rate of 0.116 h−1 and yield coefficient of 1.22 mg of dry weight of biomass/mg COD31. Yet another report showed the specific growth rate varying from 0.001 to 0.003 h−1 and yield coefficient varying from 0.25 to 0.75 mg of dry weight of biomass mg−1 COD42.
Characterization of biodegraded textile effluent
FTIR G. candidum-induced effluent biodegradation which was established through FTIR spectral analyses (Fig. 1). The process of biodegradation is demonstrated either by loss of absorbance peaks or by the occurrence of new peaks43,44. The FTIR spectrum of the untreated effluent represented the variable stretching vibrations of C = C (alkyne), P–H (phosphine) and N–H(amine) at 2127 cm−1, 2360 cm−1 and 3348 cm−1 respectively. The biodegradation products obtained after 18 h of treatment showed disappearance of phosphine group and occurrence of a new peak at 530 cm−1, representing the occurrence of strong stretching vibration of C–Br (alkyl bromide) (Fig. 1). The biodegradation of raw textile effluent by G. candidum was clearly established in this study. Previous studies have established that the biodegradation of textile dyes can be confirmed by FTIR spectrum representing occurrence of new peaks44. The signal in IR at 1634 cm−1, which corresponds to aldehyde. Thus aldehyde, one of the intermediate, formed during degradation of acid orange is confirmed. During the degradation there is asymmetric cleavage of azo bond in acid orange resulting in formation of nitrosobenzene, which was confirmed by the standard GC–MS library data, this is further converted to aniline and later on aromatic ring cleavage leading to complete mineralization. While the naphthalene part of the dye was further biodegraded with opening of one ring, the formation of aldehyde as one of the intermediate is confirmed from the IR data. On the basis of above results, it can be concluded that G. candidum has ability to mineralize acid orange completely. Similar results were demonstrated by previous studies45.
Figure 1.
FT-IR spectral analyses of raw and treated textile effluent.
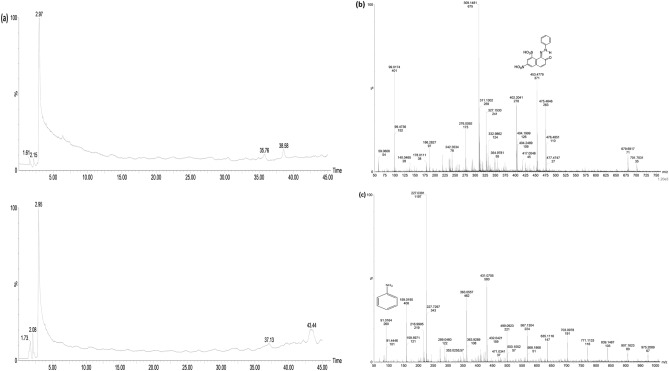
LCMS In positive ion and full scanning mode, the textile dye effluent was analyzed from 100 to 1000 m/z. Several mass peaks of varying values have been observed with both raw and treated effluent (Fig. 2a). For the raw effluent, a mass peak at 453 m/z (m.w. 452) was identical to that of an azo dye, acid orange 10 (Fig. 2b). Hence, it was evident that the acid orange 10 was a major dye component of the textile effluent used in this study. The identification of metabolites produced prior to biodegradation of effluent was carried out and the plausible biodegradation pathway based on the previous reports was predicted46. The secretion and involvement of ligninolytic enzymes (laccase and lignin peroxidase) in the azo dye degradation process of G. candidum was evident from our previous study25. The degradation of acid orange 10 by peroxidase leads to the formation of two biodegradation products such as 7-oxo-8-iminonapthalene -1,3-disulfonate and nitrosobenzene, which subsequently undergoes stepwise hydrogenation and dehydration to form aniline (m.w. 93, m/z 91) (Fig. 2c), via phenyl hydroxyl amine intermediate (as proposed by Mahata and co-workers47. The degradation pathway has been predicted in Fig. 3. Aniline was found in the treated effluent as a low molecular weight compound, rendering it less toxic.
Figure 2.
LCMS analysis of raw and treated textile effluent.
Figure 3.
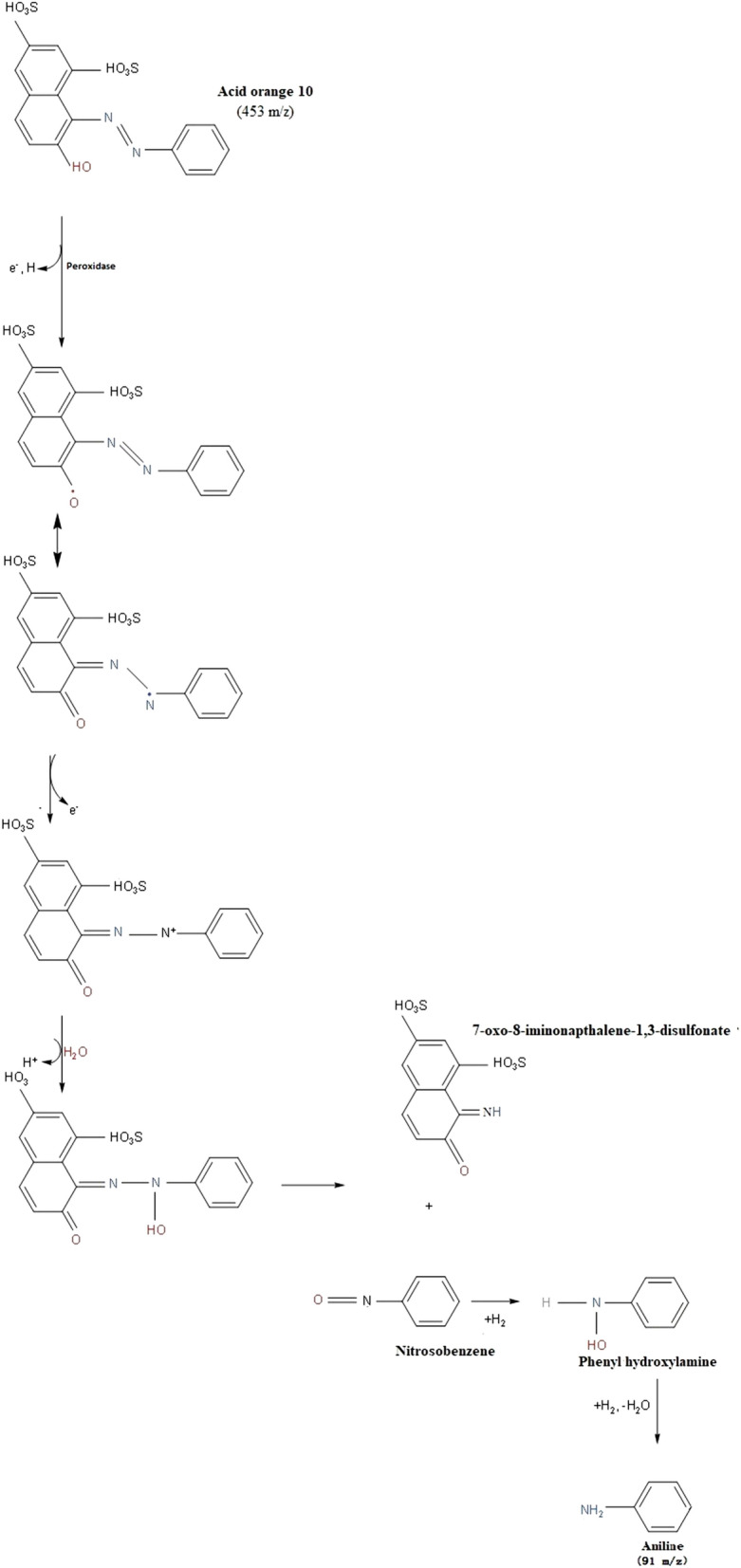
Proposed pathway for degradation of acid orange 10 dye by G. candidum (Images have been drawn using the software KingDraw (v1.1.0, http://www.kingdraw.cn/en/download.html).
XRD Heavy metal ions can be prevalent in textile effluent because of the metal-associated dyes and/or additional components used in the dyeing process. The X-ray spectra obtained for the dried samples of the untreated and treated effluent after 18 h treatment with G. candidum are shown in Fig. 4. This displayed various peaks that specifically indicated the presence of metals in raw effluent. The 2θ values of 32.36° and 50.5° show the presence of major metals, such as lead and mercury respectively. The presence of these metals was confirmed using standard JCPDS reference codes (04–0686(Pb) and 01–085-0211(Hg)). Subsequently, the X-ray spectra of treated effluent showed the absence of Pb as well as the considerable decrease in the peak intensity for Hg indicating the decreased toxicity of the effluent (Fig. 4). A decrease in % crystallinity was observed in the textile effluent after it was exposed to G. candidum, indicating the degradation of the effluent. Previous studies have demonstrated similar removal of heavy metals from textile effluent31. The presence of heavy metals in the effluent leads to numerous health hazards and sequential treatment would eliminate the heavy metals, and make the treated effluent more safe to discharge into the environment.
Figure 4.

X-ray diffraction pattern of raw and treated textile effluent.
Toxicity evaluation
Phytotoxicity
Agriculturally valuable seeds i.e. P. mungo were used for phytotoxicity assessment of raw and treated textile effluent. The analyzed parameters were germination percentage, plumule and radical length. In control set, i.e., seeds germinated in tap water, 100% germination was observed after 7 days, which reduced to about 33% in case of raw textile effluent (P > 0.05) (Fig. 5). Though some of the seeds exposed to raw textile effluent germinated, they couldn’t grow further, exhibiting maximum phytotoxicity. Similar studies on the toxic effects of azo dye-laden textile effluent on the seed germination has been reported48,49. The study on inhibitive effect of azo dyes on plants by Zhou and Xiang showed that the azo dyes inhibit the ATPase activity of plants, photosynthetic oxygen evolution and plant growth. In the current study, due to the higher degradation of textile effluent by G. candidum, a germination rate of 100% was recorded in P. mungo with treated textile effluent. It was indicative that the metabolites formed after effluent biodegradation are less harmful than the compound present in the raw textile effluent. The results shown in Table 2 indicated that the germination (%) and length of plumule and radicle of P. mungo seeds were less with the untreated as compared to treated effluent. This study showed that the seed germination and average plumule and radical development were unaffected by decolored textile effluent. A significant decrease (P > 0.05) in the average plumule and radical of the germinated seeds was found in the untreated textile effluent. Thus, the comprehensive results indicated that the textile effluent treated with G. candidum was not harmful to plant germination and growth. This ensures that treated effluent could be used for agriculture or recycled.
Figure 5.

Phytotoxicity analysis of (a) Control (Tap water), (b) Treated textile effluent, (c) Raw textile effluent on P. mungo.
Table 2.
Phytotoxicity study of untreated and treated textile effluent on P. mungo.
| Parameters | Control | Raw textile effluent | Treated textile effluent |
|---|---|---|---|
| Germination (%) | 98 ± 0.08 | 33 ± 0.06 | 95 ± 0.03 |
| Plumule (cm) Mean ± SD | 6.86 ± 0.954 | 0.28 ± 0.577 | 0.6 ± 0.441 |
| Radicle (cm) Mean ± SD | 8.38 ± 2.399 | 3.15 ± 1.755 | 6.46 ± 1.503 |
Values are mean of three experiments, SD ( ±), significantly different from the control (seeds germinated in water) at P > 0.05 (One-way analysis of variance, ANOVA).
Toxicity test with E. coli
The short-term toxicity of the raw and treated textile effluent was assessed using the well-established bacteria E. coli and the results are shown in Fig. 6. The raw textile effluent was highly toxic, while treated textile effluent presented a low toxicity. Figure 6 shows that after 30 min of exposure to raw textile effluent, the number of bacterial cells declined drastically by approximately 41%, indicating acute toxicity to E coli. This was plausibly due to the enormous amount of ionic and acid dyes entering the wastewater during the textile processing. Ionic and disperse dyes discharged from textile processing and dyeing were usually particularly toxic, and some were mutagenic and carcinogenic50,51. On the contrary, it was found that the treated effluent was absolutely harmless to the bacteria, which was evident from their uninhibited growth (cells/ml increased by approximately 10%). This indicates that the toxicity of textile effluent was reduced to a greater extent after treatment with G. candidum.
Figure 6.

Microbial toxicity analysis of (a) Control (culture medium), (b) Treated textile effluent, (c) Raw textile effluent on E. coli.
Genotoxicity analysis
The A. cepa study is a standard test to assess the genotoxicity of any toxic substance. The test was carried out to identify MI and chromosomal aberrations in the root cells (Table 3). On the basis of the MI value, (MI value can act as a biosensor for environmental contaminants) the cytotoxic effect of the toxic compound was assessed52. Table 3 shows the genotoxic aspects of the textile effluent before and after treatment. The decreased MI value is indicative of decreased cytotoxicity of treated effluent. The textile wastewater typically has a detrimental impact on chromosomal cell division, and this type of aberrations in mitotic cell division is triggered by spindle apparatus proteins malfunctioning32,53,54 or probably due to decrease in ATP synthesis during cell division. The significant reduction in COD level could then contribute to the decline in the number of aberrant mitotic cells after treatment. The findings recorded were similar to the literature data52,54.
Table 3.
Genotoxicity analysis for the raw and treated effluent.
| Analysis | Raw Effluent | Treated effluent |
|---|---|---|
| RL (cm) | 3.28 ± 0.65 | 5.84 ± 0.41 |
| MI | 0.3 ± 1.32 | 0.9 ± 0.562 |
| MN | Not found | Not found |
| CB | 3 | 1 |
| TA | 3 | 1 |
| TCA | 50 | 50 |
| Frequency of TA | 0.5 ± 0.04* | 0.25 ± 0.005 |
RL- root length; MI- Mitotic index; MN- micronuclei; CB- Chromosome breaks; TCA- total no. of cells analysed; TA-Total no. of alterations. Values are mean of three experiments, * P < 0.05, ** P < 0.001 by one-way analysis of variance (ANOVA) with Tukey–Kramer comparison test.
The decrease in colour, COD and BOD might have led to the minimization in the toxicity of textile effluent. This study indicates that the metabolites produced after biodegradation are less toxic than the compounds present in raw effluent.
Relationship between toxicity and COD of raw and treated textile effluent
Pearson’s correlation analysis for the textile effluent indicated a significantly positive correlation between COD and TU50 (r = 0.920, P < 0.05, R2 = 0.84), which suggested that compounds present in the textile effluent were toxic to E. coli. However, there was a negative relationship between COD and TU50 (r = 0.088, P = 0.048, R2 = 0.77) in case of treated textile effluent. Similar toxicity and COD correlation studies for textile effluent have been carried out with Vibrio fischeri and Desmodesmus subspicatus. They showed that there was a significant positive correlation between V. fischeri and COD; color and TU50 (r = 0.824, 0.57, P < 0.05), which suggested that compounds producing color might be toxic for V. fischeri. However, there was a negative relationship between D. subspicatus and TU50 (r = 0.625, P = 0.035)55.
COD is one of the most widely used water quality monitoring metrics and also an important measure for the regulation of the usage of wastewater treatment facilities, taxation and surveillance of wastewater effluent56.Therefore, it was important to establish associations between bio-toxicity and conventional markers such as COD57.
Conclusion
The competent Geotrichum candidum culture involved in the current work biodegraded the toxic textile effluent. The analysis of conventional parameters such as COD, BOD and color were indicative of the decreased toxicity of the treated effluent in comparison to the raw effluent. The effective decolorization and biodegradation of effluent in 18 h was confirmed by FTIR and XRD analysis. The plausible biodegradation pathway has been proposed on the basis of metabolites identified by LCMS. This demonstrated the first report on the proposed pathway for enzymatic degradation study of acid orange 10 by G. candidum. The genotoxicity, phytotoxicity and microbial toxicity analyses proved the raw effluent is harmful, whereas the treated effluent is less toxic. Relationship between effluent COD and TU50 showed that an increase in effluent COD resulted in increase in wastewater toxicity. There was a clearly defined correlation between toxicity and COD. It was evident that toxic effects of the textile effluent were significantly reduced upon treatment with G. candidum. The major relationships between toxicity and COD will provide directions for more efficient control of textile dyeing effluents. This is the first report on the positive correlation between toxicity and COD of textile effluent using G. candidum within a very short stretch of time (18 h). Therefore, the findings of this study have shown that the treated effluent was safer to be released in regard to physicochemical parameters and toxicity unit (TU50). The correlation between conventional indicators and toxicity may provide assistance in effluent management.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors have their consent for the publication of this manuscript if accepted.
Acknowledgements
This study has been funded by the Department of Science and Technology (DST/SSTP/Odisha/443) and is greatly acknowledged. We are grateful to the Center for Biotechnology, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, for their support and encouragement. We also greatly acknowledge SAIF Chandigarh for their support in LCMS analysis.
Funding
This study has been funded by the Department of Science and Technology (DST/SSTP/Odisha/443) and is greatly acknowledged.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Declarations
Competing interests
Not applicable.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agrawal K, Verma P. Biodegradation of synthetic dye Alizarin Cyanine Green by yellow laccase producing strain Stropharia sp. ITCC-8422. Biocatal. Agric. Biotechnol. 2019;21:101291. doi: 10.1016/j.bcab.2019.101291. [DOI] [Google Scholar]
- 2.Kumar V, Upadhyay N, Singh S, Singh J, Kaur P. Thin-layer chromatography: Comparative estimation of Soil’s atrazine. Curr. World Environ. J. 2013;8:469–472. doi: 10.12944/CWE.8.3.17. [DOI] [Google Scholar]
- 3.Kumar V, et al. Bioremediation of Petroleum hydrocarbon by using Pseudomonas species isolated from Petroleum contaminated soil. Orient. J. Chem. 2014;30:1771–1776. doi: 10.13005/ojc/300436. [DOI] [Google Scholar]
- 4.Kumar V, et al. Bioremediation of heavy metals by employing resistant microbial isolates from agricultural soil irrigated with Industrial Waste water. Orient. J. Chem. 2015;31:357–361. doi: 10.13005/ojc/310142. [DOI] [Google Scholar]
- 5.Kumar V, Singh S, Singh J, Upadhyay N. Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull. Environ. Contam. Toxicol. 2015;94:807–814. doi: 10.1007/s00128-015-1523-7. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Balomajumder C. Simultaneous biosorption and bioaccumulation of phenol and cyanide using coconut shell activated carbon immobilized Pseudomonas putida (MTCC 1194) J. Environ. Chem. Eng. 2016;4:1604–1614. doi: 10.1016/j.jece.2016.02.011. [DOI] [Google Scholar]
- 7.Mishra V, et al. Synergistic effects of Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in bioremediation of iron contaminated soils. Int. J. Phytoremediation. 2016;18:697–703. doi: 10.1080/15226514.2015.1131231. [DOI] [PubMed] [Google Scholar]
- 8.Reddy S, Osborne JW. Biodegradation and biosorption of Reactive Red 120 dye by immobilized Pseudomonas guariconensis: Kinetic and toxicity study. Water Environ. Res. 2020;92:1230–1241. doi: 10.1002/wer.1319. [DOI] [PubMed] [Google Scholar]
- 9.Hamidi R, M., Jovanova, B. & Kadifkova Panovska, T. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014;60:9–18. doi: 10.33320/maced.pharm.bull.2014.60.01.002. [DOI] [Google Scholar]
- 10.Kumar V, Singh S, Singh R, Upadhyay N, Singh J. Design, synthesis, and characterization of 2,2-bis(2,4-dinitrophenyl)-2-(phosphonatomethylamino)acetate as a herbicidal and biological active agent. J. Chem. Biol. 2017;10:179–190. doi: 10.1007/s12154-017-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, et al. Toxicity, degradation and analysis of the herbicide atrazine. Environ. Chem. Lett. 2018;16:211–237. doi: 10.1007/s10311-017-0665-8. [DOI] [Google Scholar]
- 12.Singh S, et al. Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and Fe(III)], and humic acid. 3 Biotech. 2017;7:262. doi: 10.1007/s13205-017-0900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzeo DEC, Fernandes TCC, Marin-Morales MA. Cellular damages in the Allium cepa test system, caused by BTEX mixture prior and after biodegradation process. Chemosphere. 2011;85:13–18. doi: 10.1016/j.chemosphere.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y, Lo T, Chou H, Laio YL. Anaerobic biodegradation of decabromodiphenyl ether (BDE-209)-contaminated sediment by organic compost. Int. Biodeterior. Biodegradation. 2016;113:228–237. doi: 10.1016/j.ibiod.2016.04.017. [DOI] [Google Scholar]
- 15.Barnes RJ, Molina R, Xu J, Dobson PJ, Thompson IP. Comparison of TiO2 and ZnO nanoparticles for photocatalytic degradation of methylene blue and the correlated inactivation of gram-positive and gram-negative bacteria. J. Nanoparticle Res. 2013;15:1432. doi: 10.1007/s11051-013-1432-9. [DOI] [Google Scholar]
- 16.Ghosh M, Chakraborty A, Mukherjee A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO 2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J. Appl. Toxicol. 2013;33:1097–1110. doi: 10.1002/jat.2863. [DOI] [PubMed] [Google Scholar]
- 17.Goncalves DM, Girard D. Zinc oxide nanoparticles delay human neutrophil apoptosis by a de novo protein synthesis-dependent and reactive oxygen species-independent mechanism. Toxicol. Vitr. 2014;28:926–931. doi: 10.1016/j.tiv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Leung YH, et al. Toxicity of CeO2 nanoparticles: The effect of nanoparticle properties. J. Photochem. Photobiol. B Biol. 2015;145:48–59. doi: 10.1016/j.jphotobiol.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Davies LC, Pedro IS, Novais JM, Martins-Dias S. Aerobic degradation of acid orange 7 in a vertical-flow constructed wetland. Water Res. 2006;40:2055–2063. doi: 10.1016/j.watres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Wanderley CRP, Andrade MV, Pereira LJ, Silva GMM, Pessoa KR. Azo dye mineralization by Phanerochaete Chysosporium in a sequencing bath reactor. Braz. Arch. Biol. Technol. 2018;2018:61. [Google Scholar]
- 21.SeongJun Kim; Makoto Shoda Decolorization of molasses by a new isolate of Geotrichum candidum in a jar fermenter. Biotechnol. Tech. 1998;12:497–499. doi: 10.1023/A:1008824119174. [DOI] [Google Scholar]
- 22.Kim SJ, Shoda M. Decolorization of molasses and a dye by a newly isolated strain of the fungusGeotrichum candidum Dec 1. Biotechnol. Bioeng. 1999;62:114–119. doi: 10.1002/(SICI)1097-0290(19990105)62:1<114::AID-BIT13>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Shintani N, Shoda M. Decolorization of oxygen-delignified bleaching effluent and biobleaching of oxygen-delignified kraft pulp by non-white-rot fungus Geotrichum candidum Dec 1. J. Environ. Sci. 2013;25:S164–S168. doi: 10.1016/S1001-0742(14)60649-5. [DOI] [PubMed] [Google Scholar]
- 24.Yin L, Chen Z-H, Zhao S-J. Optimization of laccase production from Geotrichum candidum and decoloration of azo dyes by laccase. Res Square. 2008;36:85–90. [Google Scholar]
- 25.Rajhans G, Sen SK, Barik A, Raut S. Elucidation of fungal dye-decolourizing peroxidase (DyP) and ligninolytic enzyme activities in decolourization and mineralization of azo dyes. J. Appl. Microbiol. 2020;129:1633–1643. doi: 10.1111/jam.14731. [DOI] [PubMed] [Google Scholar]
- 26.Rajhans G., Sen S.K., Barik A., Raut S. De‐colorization of textile effluent using immobilized Geotrichum candidum : An insight into mycoremediation. Lett. Appl. Microbiol. 2020;72(4):445–457. doi: 10.1111/lam.13430. [DOI] [PubMed] [Google Scholar]
- 27.Yu W, Liu W, Huang H, Zheng F, Wang X, Wu Y, et al. Application of a novel alkali-tolerant thermostable dyp-type peroxidase from Saccharomonospora viridis DSM 43017 in biobleaching of eucalyptus kraft pulp. PLoS ONE. 2014;9:110319. doi: 10.1371/journal.pone.0110319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma K, Qin Z, Zhao Z, Zhao C, Liang S. Toxicity evaluation of wastewater collected at different treatment stages from a pharmaceutical industrial park wastewater treatment plant. Chemosphere. 2016;158:163–170. doi: 10.1016/j.chemosphere.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 29.Rice E.W., Baird R.B., Eaton A.D. APHA Standard Methods For The Examination Of Water And Wastewater. 23. American Public Health Association, American Water Works Association, Water Environment Federation; 2017. [Google Scholar]
- 30.Tufekci N, San H.A., Aydin S., Ucar S., Barlas H. Wastewater Treatment Problems in the Operation of Woven and Knit Fabric Industry. Fed. Eur. Biochem. Soc. 1998;7:795–802. [Google Scholar]
- 31.Vijayalakshmidevi SR, Muthukumar K. Improved biodegradation of textile dye effluent by coculture. Ecotoxicol. Environ. Saf. 2015;114:23–30. doi: 10.1016/j.ecoenv.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Wijeyaratne WMDN, Wadasinghe LGYJG. Allium cepa Bio Assay to Assess the Water and Sediment Cytogenotoxicity in a Tropical Stream Subjected to Multiple Point and Nonpoint Source Pollutants. J. Toxicol. 2019;2019:1–10. doi: 10.1155/2019/5420124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norberg-King Teresa J. A linear interpolation method for sublethal toxicity: The inhibition concentration (ICp) approach (version 2.0) 2. Minnesota: USEPA; 1993. pp. 03–93. [Google Scholar]
- 34.Huber W, Koella JC. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993;55:257–261. doi: 10.1016/0001-706X(93)90083-N. [DOI] [PubMed] [Google Scholar]
- 35.Alexander B, Browse DJ, Reading SJ, Benjamin IS. A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Methods. 1999;41:55–58. doi: 10.1016/S1056-8719(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, et al. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca Sepium and its main component 2-hydroxy-4-methoxybenzaldehyde. Molecules. 2010;15:5807–5817. doi: 10.3390/molecules15085807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprague JB. Measurement of pollutant toxicity to fish. II. Utilizing and applying bioassay results. Water Res. 1970;4:3–32. doi: 10.1016/0043-1354(70)90018-7. [DOI] [Google Scholar]
- 38.Yaseen DA, Scholz M. Shallow pond systems planted with Lemna minor treating azo dyes. Ecol. Eng. 2016;94:295–305. doi: 10.1016/j.ecoleng.2016.05.081. [DOI] [Google Scholar]
- 39.Islam MR, Mostafa MG. Characterization of textile dyeing effluent and its treatment using polyaluminum chloride. Appl. Water Sci. 2020;10:119. doi: 10.1007/s13201-020-01204-4. [DOI] [Google Scholar]
- 40.Gurbuz F, Ozcan A, Ciftci H, Acet O, Odabasi M. Treatment of textile effluents through bio-composite column: Decolorization and COD reduction. Int. J. Environ. Sci. Technol. 2019;16:8653–8662. doi: 10.1007/s13762-019-02430-3. [DOI] [Google Scholar]
- 41.Bankole PO, Adekunle AA, Obidi OF, Chandanshive VV, Govindwar SP. Biodegradation and detoxification of Scarlet RR dye by a newly isolated filamentous fungus, Peyronellaea prosopidis. Sustain. Environ. Res. 2018;28:214–222. doi: 10.1016/j.serj.2018.03.001. [DOI] [Google Scholar]
- 42.Bayram TT, Nuhoğlu A, Aladağ E. Investigation of biodegradation and growth kinetics of dairy wastewater in a batch reactor. Bulg. Chem. Commun. 2017;49:896–900. [Google Scholar]
- 43.Chen K-C, Wu J-Y, Liou D-J, Hwang S-CJ. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 2003;101:57–68. doi: 10.1016/S0168-1656(02)00303-6. [DOI] [PubMed] [Google Scholar]
- 44.Sen SK, Patra P, Das CR, Raut S, Raut S. Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: An impact on its use in irrigation of wheat crop. Water Resour. Ind. 2019;21:100106. doi: 10.1016/j.wri.2019.100106. [DOI] [Google Scholar]
- 45.Shah MP. Bioremedial application of bacillus megaterium PMS82 in microbial degradation of acid orange dye. Int. J. Environ. Bioremediation Biodegrad. 2014;2:93–99. [Google Scholar]
- 46.Chacko JT, Subramaniam K. Enzymatic degradation of azo dyes: A review. Int. J. Environ. Sci. 2011;1:1250–1260. [Google Scholar]
- 47.Mahata A, Rai RK, Choudhuri I, Singh SK, Pathak B. Direct vs. indirect pathway for nitrobenzene reduction reaction on a Ni catalyst surface: a density functional study. Phys. Chem. Chem. Phys. 2014;16:26365–26374. doi: 10.1039/C4CP04355C. [DOI] [PubMed] [Google Scholar]
- 48.Nasrin T, Saha A, Mohanta M, Chaity, A& Rahman, S & Ruhi, R & Sarker, S & Haque, M. Decolourization of azo dye by indigenous bacteria and its impact on seed germination. Int. J. Biosci. 2019;14:197–210. [Google Scholar]
- 49.Zhou X, Xiang X. Effect of different plants on azo-dye wastewater bio-decolorization. Procedia Environ. Sci. 2013;18:540–546. doi: 10.1016/j.proenv.2013.04.073. [DOI] [Google Scholar]
- 50.Li J, Shao Z, Chen C, Wang X. Hierarchical GOs/Fe 3 O 4 /PANI magnetic composites as adsorbent for ionic dye pollution treatment. RSC Adv. 2014;4:38192. doi: 10.1039/C4RA05800C. [DOI] [Google Scholar]
- 51.Saini RD. Textile organic dyes: Polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 2017;9:121–136. [Google Scholar]
- 52.Caritá R, Marin-Morales MA. Induction of chromosome aberrations in the Allium cepa test system caused by the exposure of seeds to industrial effluents contaminated with azo dyes. Chemosphere. 2008;72:722–725. doi: 10.1016/j.chemosphere.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 53.Wijeyaratne WMDN, Wickramasinghe PGMU. Chromosomal abnormalities in allium cepa induced by treated textile effluents: Spatial and temporal variations. J. Toxicol. 2020;2020:1–10. doi: 10.1155/2020/8814196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jadhav JP, Kalyani DC, Telke AA, Phugare SS, Govindwar SP. Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresour. Technol. 2010;101:165–173. doi: 10.1016/j.biortech.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 55.Liang J, et al. Toxicity evaluation of textile dyeing effluent and its possible relationship with chemical oxygen demand. Ecotoxicol. Environ. Saf. 2018;166:56–62. doi: 10.1016/j.ecoenv.2018.08.106. [DOI] [PubMed] [Google Scholar]
- 56.Zheng Q, et al. Self-organized TiO2 nanotube array sensor for the determination of chemical oxygen demand. Adv. Mater. 2008;20:1044–1049. doi: 10.1002/adma.200701619. [DOI] [Google Scholar]
- 57.Gholami-Borujeni F, Nejatzadeh-Barandozi F, Aghdasi H. Data on effluent toxicity and physicochemical parameters of municipal wastewater treatment plant using Daphnia Magna. Data Br. 2018;19:1837–1843. doi: 10.1016/j.dib.2018.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.