Abstract
Introduction
Plasma volume status (PVS), a parameter of the discrepancy between actual plasma volume (PV) and ideal PV, has been recently evaluated as a prognostic marker in patients with heart failure. This subgroup analysis of the EMBODY trial was designed to determine whether a sodium-glucose cotransporter 2 (SGLT2) inhibitor affects the alleviation of heart failure and improvement of PVS in patients after acute myocardial infarction (AMI) with congestive heart failure (CHF).
Methods
The EMBODY trial was a prospective, multicenter, randomized, double-blind, placebo-controlled trial to identify the effect of an SGLT2 inhibitor on cardiac sympathetic hyperactivity in patients with AMI and type 2 diabetes mellitus (T2DM) in Japan. In total, 105 patients were randomized (1:1) to receive 10 mg empagliflozin or a placebo (once daily), 2 weeks after the onset of AMI. In this subanalysis, we investigated the time-course of PVS at baseline and weeks 4, 12, and 24.
Results
Overall, 96 patients were included in the subgroup analysis set (age 64.3 ± 10.9 years, 80.2% men; 46 in the empagliflozin group and 50 in the placebo group). Body weight and PVS decreased in the empagliflozin group compared with the placebo group at 24 weeks (− 2.2 vs. + 0.1 kg, P < 0.001, and − 5.1 vs. − 0.3%, P < 0.001, respectively). Decreased PVS, defined as a change in PVS of < − 4.5%, was associated with the administration of empagliflozin (odds ratio 2.61, 95% confidence interval 1.11–6.15, P = 0.028). N-terminal pro b-type natriuretic peptide levels decreased in both the empagliflozin and placebo groups (1028.7–370.3 pg/mL, P < 0.001, and 1270.6–673.7 pg/mL, P < 0.01, respectively).
Conclusion
Empagliflozin reduced the body weight and PVS. Early SGLT2 inhibitor administration in patients with AMI, CHF, and T2DM can therefore be effective in reducing the body weight and PVS.
Trial Registration
UMIN 000030158.
Keywords: Acute myocardial infarction, Congestive heart failure, Empagliflozin, Plasma volume status, Sodium-glucose cotransporter 2 (SGLT2) inhibitor
Key Summary Points
| Why carry out this study? |
| Plasma volume status (PVS), an indication of the discrepancy between actual plasma volume (PV) and ideal PV, has been recently evaluated as a prognostic marker in patients with heart failure. |
| This trial was designed to determine whether a sodium-glucose cotransporter 2 (SGLT2) inhibitor (empagliflozin) affected the improvement of heart failure and PVS in patients after acute myocardial infarction (AMI) with congestive heart failure (CHF). |
| What was learned from the study? |
| Empagliflozin reduced not only the body weight but also PVS. |
| The results suggest that early SGLT2 inhibitor administration in patients with AMI, CHF, and T2DM can be effective in reducing the body weight and PVS. |
Introduction
Plasma volume status (PVS) is a differential marker used in the case of a discrepancy between actual plasma volume (PV) and ideal PV; it has recently been evaluated as a prognostic marker of heart failure [1, 2]. Actual PV has been validated previously [3] and is derived from the values of the hematocrit and the weight of subjects compared with directly measured values of PV, using the curve fitting technique.
| 1 |
where hematocrit is a fraction, a = 1530 in males and 864 in females, and b = 41 in males and 47.9 in females.
Using the body weight, the ideal PV is calculated from the following well-established formula [1]:
| 2 |
where c = 39 in males and 40 in females.
Subsequently, relative PVS, an index of the degree of deviation in ideal PV, can be calculated using the Kaplan–Hakim formula as follows [1]:
| 3 |
The EMBODY trial is a prospective, multicenter, randomized, double-blind, and placebo-controlled trial for identifying the effect of empagliflozin, a sodium-glucose cotransporter 2 (SGLT2) inhibitor, on cardiac sympathetic hyperactivity in patients with acute myocardial infarction (AMI) and type 2 diabetes mellitus (T2DM) in Japan [4, 5].
This post hoc analysis of the EMBODY trial was designed to determine whether the SGLT2 inhibitor improves PVS in patients with diabetes with congestive heart failure (CHF) after AMI.
Methods
In the subgroup analysis of the EMBODY trial, a total of 105 patients with T2DM were randomized (1:1) to receive 10 mg empagliflozin once daily or the placebo, both within 2 weeks of the onset of AMI. Here, we investigated the time-course of changes in PVS at baseline and at weeks 4, 12, and 24. All patients provided informed consent to participate in the trial between February 2018 and March 2019. The inclusion and exclusion criteria have already been published [3]. Six patients in the empagliflozin group and three patients in the placebo group withdrew their consent and were thus excluded before the medication was initiated.
Ethics
The EMBODY trial was registered at the UMIN in November 2017 (ID: 000030158). Ethics approval was obtained from the local institutional review board of each participating center. The trial was conducted in full compliance with the articles of the Declaration of Helsinki and according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Health, Labour, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology in Japan. After initial screening for eligibility using prior medical records, each patient received an adequate explanation of the trial plan before they provided written informed consent. All participants provided written informed consent prior to study enrolment.
Results
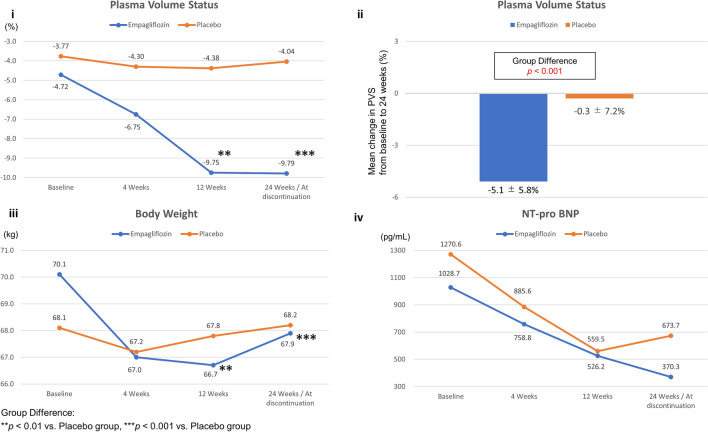
In total, 96 patients were included in the subgroup analysis set (age 64.3 ± 10.9 years, 80.2% men, with 46 and 50 patients in the empagliflozin and placebo groups, respectively). The baseline characteristics were not significantly different between the treatment groups, including left ventricular ejection fraction, and New York Heart Association functional classification (Table 1). During the trial period, no significant differences were observed in terms of the dose and oral administration rate of the optimal medical treatment (including diuretics) between the two groups (Table 2). All patients received both revascularization and heart failure treatments. The empagliflozin group showed significantly decreased body weight and PVS compared with the placebo group at 24 weeks (− 2.2 vs. + 0.1 kg, P < 0.001, and − 5.1 ± 5.8% vs. − 0.3 ± 7.2%, P < 0.001, respectively) (Fig. 1a–c; Table 2). Therefore, an intergroup difference was observed at 12 and 24 weeks (Fig. 1a, c). On the contrary, the N-terminal pro b-type natriuretic peptide (NT-proBNP) level was decreased in both groups (1028.7–370.3 pg/mL, P < 0.001, and 1270.6–673.7 pg/mL, P < 0.01, respectively), and intergroup differences were not significant (P = 0.91) (Fig. 1d; Table 2). Blood test results are presented in Table 2 During the trial period, no difference was observed between the groups in terms of glycated hemoglobin (HbA1c) levels. Compared to the placebo group, the empagliflozin group exhibited significantly higher hematocrit levels and lower uric acid levels at 24 weeks. The decrease in PVS was defined as a change of < − 4.5%, which was the median value calculated using the change in PVS (ΔPVS) of patients from baseline to 24 weeks. Univariate and multivariate logistic regression analyses adjusted for confounding variables (age, NT-proBNP levels, change in body weight, systolic blood pressure, HbA1c levels) indicated that the decrease in PVS was associated with empagliflozin use (odds ratio [OR] 2.61, 95% confidence interval (CI) 1.11–6.15, P = 0.028, and OR 3.76, 95% CI 1.11–12.72, P = 0.033 for univariate and multivariate models, respectively) (Table 3). Additionally, ΔPVS was not significantly correlated with the change in NT-proBNP (ΔNT-proBNP) in both the empagliflozin (r = 0.194, P = 0.289) and placebo (r = 0.254, P = 0.109) groups.
Table 1.
Baseline patient characteristics
| Patient characteristics | Empagliflozin group, n = 46 | Placebo group, n = 50 | P |
|---|---|---|---|
| Age, year (SD) | 63.9 (10.4) | 64.6 (11.6) | 0.73 |
| Male, n (%) | 38 (82.6) | 39 (78.0) | 0.62 |
| Body weight, kg (SD) | 70.1 (13.7) | 68.1 (14.4) | 0.49 |
| BMI, kg/m2 (SD) | 25.2 (3.7) | 25.2 (4.1) | 0.99 |
| Systolic blood pressure, mmHg (SD) | 129.7 (11.9) | 123.1 (15.7) | 0.11 |
| Hypertension, n (%) | 38 (82.6) | 39 (78.0) | 0.62 |
| Dyslipidemia, n (%) | 34 (73.9) | 36 (72.0) | 1.00 |
| Smoking, n (%) | 24 (52.2) | 27 (54.0) | 0.92 |
| Transthoracic echocardiography | |||
| Left ventricular ejection fraction, % (SD) | 55.1 (14.2) | 55.0 (13.7) | 0.97 |
| Average E/e′ (SD) | 13.47 (7.24) | 13.73 (6.26) | 0.88 |
| TRPG, mmHg (SD) | 17.59 (7.48) | 20.71 (11.49) | 0.23 |
| Heart failure medication at randomization visit, n (%) | |||
| β-Blocker | 41 (89.1) | 38 (76.0) | 0.11 |
| Diuretic | 8 (17.4) | 11 (22.0) | 0.62 |
| MRA, | 11 (23.9) | 12 (24.0) | 1.00 |
| ACEI | 23 (50.0) | 28 (56.0) | 0.68 |
| ARB | 22 (47.8) | 19 (38.0) | 0.41 |
| Glucose-lowering medication at randomization visit, n (%) | |||
| Biguanide | 7 (15.2) | 6 (12.0) | 0.77 |
| DPP-4 inhibitor | 20 (43.5) | 23 (46.0) | 0.84 |
| NYHA classification, % | |||
| I/II | 77.8/7.4 | 58.1/22.6 | 0.09 |
| Blood test | |||
| Max CK, IU/L (SD) | 2080.7 (2461.6) | 2358.7 (2829.1) | 0.61 |
| HbA1c, % (SD) | 6.82 (1.00) | 6.89 (0.92) | 0.73 |
| LDL-C, mg/dL (SD) | 87.5 (29.6) | 87.8 (29.1) | 0.96 |
| HDL-C, mg/dL (SD) | 45.4 (12.7) | 46.0 (10.2) | 0.78 |
| Triglycerides, mg/dL (SD) | 161.7 (119.7) | 135.9 (54.4) | 0.18 |
| Uric acid, mg/dL (SD) | 5.8 (1.4) | 5.7 (1.5) | 0.94 |
| Creatinine, mg/dL (SD) | 0.92 (0.2) | 0.92 (1.2) | 0.39 |
| eGFR, mL/min/1.73 m2 (SD) | 64.6 (15.0) | 66.1 (15.7) | 0.62 |
| Hematocrit, % (SD) | 40.5 (4.6) | 40.3 (4.2) | 0.86 |
| NT-proBNP, pg/mL (SD) | 1028.7 (1105.6) | 1270.6 (1521.0) | 0.45 |
A1c glycated hemoglobin, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin-receptor blocker, BMI body mass index, CK creatine kinase, DPP-4 dipeptidyl peptidase-4, E peak early diastolic phase mitral inflow velocity, e′ mitral annular velocity, eGFR estimated glomerular filtration rate, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, MRA mineralocorticoid receptor antagonist, NT-proBNP N-terminal pro b-type natriuretic peptide, NYHA New York Heart Association, SD Standard deviation, TRPG trans-tricuspid pressure gradient
Table 2.
Blood tests, optimal medical therapy, and plasma volume status in the empagliflozin and placebo groups at baseline and 24 weeks
| Blood tests, optimal medical therapy, and PVS | Empagliflozin group (n = 46) | Placebo group (n = 50) | Intergroup | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 24 weeks | P | Baseline | 24 weeks | P | P | |
| Blood tests | |||||||
| HbA1c (%) | 6.8 ± 1.0 | 6.6 ± 0.9 | 0.10 | 6.9 ± 0.9 | 6.8 ± 1.0 | 0.34 | 0.50 |
| Hematocrit (%) | 40.5 ± 4.6 | 44.2 ± 3.9 | < 0.001 | 40.3 ± 4.2 | 40.5 ± 4.2 | 0.81 | < 0.0001 |
| Uric acid (mg/dL) | 5.8 ± 1.4 | 4.9 ± 1.4 | < 0.001 | 5.7 ± 1.5 | 5.8 ± 1.5 | 0.82 | < 0.0001 |
| eGFR (equivalent) (mL/min/1.73 m2) | 64.6 ± 15.0 | 64.4 ± 16.8 | 0.84 | 66.1 ± 15.7 | 62.8 ± 15.4 | 0.02 | 0.10 |
| Serum creatinine (mg/dL) | 0.92 ± 0.19 | 0.94 ± 0.25 | 0.44 | 0.89 ± 0.20 | 0.94 ± 0.21 | 0.01 | 0.24 |
| NT-proBNP (pg/mL) | 1028.7 ± 1105.6 | 370.3 ± 530.9 | < 0.001 | 1270.6 ± 1521.0 | 673.7 ± 1151.1 | < 0.01 | 0.91 |
| LDL-C (mg/dL) | 87.5 ± 29.6 | 79.4 ± 21.3 | 0.06 | 87.8 ± 29.1 | 81.0 ± 25.0 | 0.09 | 0.83 |
| HDL-C (mg/dL) | 45.4 ± 12.7 | 49.8 ± 13.0 | < 0.001 | 46.0 ± 10.2 | 49.2 ± 9.2 | 0.01 | 0.52 |
| TG (mg/dL) | 161.7 ± 119.7 | 145.5 ± 75.0 | 0.25 | 135.9 ± 54.3 | 141.0 ± 59.1 | 0.67 | 0.30 |
| AST (U/L) | 24.7 ± 7.9 | 23.2 ± 8.5 | 0.30 | 24.4 ± 10.3 | 25.4 ± 14.0 | 0.47 | 0.21 |
| ALT (U/L) | 26.4 ± 14.8 | 22.2 ± 13.1 | 0.04 | 26.6 ± 16.4 | 24.2 ± 11.9 | 0.20 | 0.54 |
| γ-GTP (U/L) | 39.6 ± 29.1 | 40.0 ± 40.2 | 0.92 | 32.4 ± 18.9 | 39.2 ± 38.1 | 0.03 | 0.16 |
| Optimal medical therapy | |||||||
| β-Blocker, n (%) | 41 (89.1) | 41 (89.1) | 1.00 | 38 (76) | 38 (76) | 1.00 | 0.09 |
| Diuretic, n (%) | 8 (17.4) | 6 (13.0) | 0.57 | 11 (22) | 10 (20) | 0.81 | 0.36 |
| MRA, n (%) | 11 (23.9) | 11 (23.9) | 1.00 | 12 (24) | 12 (24) | 1.00 | 0.99 |
| ACEI, n (%) | 23 (50) | 20 (43.5) | 0.54 | 28 (56) | 22 (44) | 0.23 | 0.96 |
| ARB, n (%) | 22 (47.8) | 21 (45.7) | 0.84 | 19 (38) | 19 (38) | 1.00 | 0.45 |
| PVS | |||||||
| PVS (%) | − 4.72 ± 8.95 | − 9.79 ± 8.07 | < 0.001 | − 3.77 ± 9.64 | − 4.04 ± 10.12 | 0.79 | < 0.001 |
ALT Alanine aminotransferase, AST aspartate aminotransferase, PVS plasma volume status, TG triglycerides, γ-GTP γ-glutamyl transpeptidase
Fig. 1.
i Time-course of change in plasma volume status (PVS) from baseline to 24 weeks. ii Change in PVS between baseline and 24 weeks. iii Time-course of change in body weight from baseline to 24 weeks. iv Time-course of change in N-terminal pro b-type natriuretic peptide (NT-proBNP) levels from baseline to 24 weeks
Table 3.
Odds ratio associated with ΔPVS < − 4.5%
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Empagliflozin | 2.61 (1.11–6.15) | 0.028 | 3.76 (1.11–12.72) | 0.033 |
| Age | 1.00 (0.96–1.04) | 0.931 | ||
| NT-proBNP | 1.00 (1.00–1.01) | 0.355 | ||
| ΔBody weight | 0.97 (0.86–1.09) | 0.588 | ||
| ΔSystolic blood pressure | 1.01 (0.98–1.03) | 0.697 | ||
| ΔHbA1c levels | 0.99 (0.58–1.70) | 0.977 | ||
CI Confidence interval, OR odds ratio, Δ delta
Discussion
A previous study reported that a PVS > − 4% was associated with the worst prognosis [1]. Here, the NT-proBNP levels were significantly decreased in both the empagliflozin and placebo groups. Moreover, ΔPVS had no correlation with ΔNT-proBNP in both groups. In the previous study, canagliflozin, another SGLT2 inhibitor, showed no robust effects on NT-proBNP levels in patients with T2DM and CHF [6]. Moreover, the DEFINE-HF trial showed that in patients with heart failure and reduced ejection fraction with and without T2DM, the use of dapagliflozin over 12 weeks did not affect the mean NT-proBNP level; however an increased proportion of patients on this drug experienced clinically meaningful improvements in the heart failure-related health status, according to the Kansas City Cardiomyopathy Questionnaire overall summary score [7]. We showed that empagliflozin reduced circulating PV independent of NT-proBNP levels. The lack of correlation between ΔPVS and ΔNT-proBNP is similar to that observed upon valsartan administration in the Heart Failure Trial [1]. The reason for such a result is unclear, and further research is warranted. The novelty of the current study is that early administration of empagliflozin in patients with AMI and T2DM reduced PVS over a 24-week period. As this study corresponds to a subanalysis, a large-scale, randomized, controlled trial with PVS as the primary endpoint is required in the future to confirm our results.
This study has several limitations. First, the trial period was only 24 weeks; nevertheless, no study has previously examined how PVS changes during 24 weeks after the administration of SGLT2 inhibitors. Second, our sample size was small. Third, we excluded patients treated with insulin, glucagon-like peptide 1 analogs, and high doses of sulfonylureas, as well as patients with HbA1c ≥ 10%. Therefore, this study did not include patients with poor glycemic control. Finally, although 20% of patients were women, the results were comparable even when the data of female patients were excluded from the analysis.
Conclusion
In conclusion, we showed that empagliflozin reduced circulating PV in patients with AMI and T2DM.
Acknowledgements
We wish to thank the staff and patients who participated in the EMBODY trial.
Funding
This work and the journal’s Rapid Service were supported by Boehringer Ingelheim and Eli Lilly and Company [grant number: 1245-0175]. The funding agencies had no role in designing or conducting the trial.
Editorial Assistance
We wish to thank all Editage staffs who proofread our paper in Cactus Communications (supported by Boehringer Ingelheim and Eli Lilly and Company [grant number: 1245-0175]).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
YH, KM, ST, and YT analyzed the data. YH, YK, KY, YI, TY, and TH wrote the manuscript and analyzed the data. YT, KA, and MM reviewed/edited the manuscript. YM, EK, MM, JT, and WS analyzed the data and contributed to the discussion. All authors read and approved the final manuscript.
Prior Presentation
American Heart Association Scientific Session 2020, 14 November 2020; https://www.ahajournals.org/toc/circ/142/Suppl_3.
Disclosures
Yu Hoshika, Yoshiaki Kubota, Kosuke Mozawa, Shuhei Tara, Yukichi Tokita, Kenji Yodogawa, Yu-ki Iwasaki, Takeshi Yamamoto, Hitoshi Takano, Yayoi Tsukada, Kuniya Asai, Masaaki Miyamoto, Yasushi Miyauchi, Eitaro Kodani, Mitsunori Maruyama, and Jun Tanabe declare no conflicts of interest. Wataru Shimizu has received honoraria and research grants from Boehringer Ingelheim.
Compliance with Ethics Guidelines
The EMBODY trial was registered at the UMIN in November 2017 (ID: 000030158). Ethics approval was obtained from the local institutional review board of each participating center. The trial was conducted in full compliance with the articles of the Declaration of Helsinki and according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Health, Labour, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology in Japan. After initial screening for eligibility using prior medical records, each patient received an adequate explanation of the trial plan before they provided written informed consent. All participants provided written informed consent prior to study enrolment.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Ling HZ, Flint J, Damgaard M, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17:35–43. doi: 10.1002/ejhf.193. [DOI] [PubMed] [Google Scholar]
- 2.Grodin JL, Philips S, Mullens W, et al. Prognostic implications of plasma volume status estimates in heart failure with preserved ejection fraction: Insights from TOPCAT. Eur J Heart Fail. 2019;21:634–642. doi: 10.1002/ejhf.1407. [DOI] [PubMed] [Google Scholar]
- 3.Hakim RM. Plasmapheresis. In: Daugirdas JT, Blake PG, Ing TS, editors. Handbook of dialysis. Philadelphia: Lippincott Williams and Wilkins; 2001. p. 236. [Google Scholar]
- 4.Kubota Y, Yamamoto T, Tara S, et al. Effect of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: Rationale. Diabetes Ther. 2018;9:2107–2116. doi: 10.1007/s13300-018-0480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu W, Kubota Y, Hoshika Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19:1–12. doi: 10.1186/s12933-020-01127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka A, Hisauchi I, Taguchi I, et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE) ESC Heart Fail. 2020;7:1585–1594. doi: 10.1002/ehf2.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF Trial. Circulation. 2019;140:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.