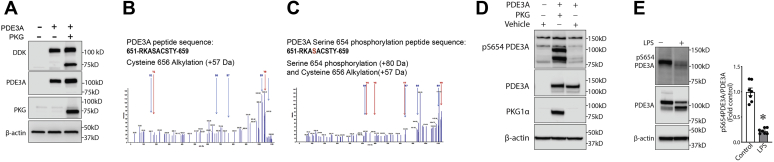
Figure 5.
PKG-Iα phosphorylates PDE3A at S654. HEK293T cells were transfected with an expression plasmid for PDE3A either alone or cotransfected with a PKG-Iα expression plasmid. Then cell lysates were subjected to PDE3A immunoprecipitation followed by SDS-PAGE and MALDI-TOF MS analysis as described in the Experimental procedures section. A, transfection and expression of PDE3A and PKG-Iα in HEK293T cells were confirmed by immunoblotting using antibodies against FLAG-tag, PDE3A, and PKG-Iα. B and C, MS/MS identified a PDE3A-specific peptide containing a serine located at position 654 (predicted PKG phosphorylation site) that was only phosphorylated when cells were transfected with PDE3A and PKG-Iα. D, the antibody generated against PKG-specific phosphorylation site, phospho-S654 in PDE3A, specifically recognized this modification when PDE3A expressed with PKG-Iα in HEK293T cells and did not react with PDE3A expressed alone. E, the antibody specific to phospho-S654-PDE3A demonstrated dramatic changes of this PDE3A modification in mouse model of ALI. Data represent mean ± SEM, n = 7. ∗p < 0.05 control versus lipopolysaccharide (LPS)-treated mice. HEK293T, human embryonic kidney 293T cells; PDE3A, phosphodiesterase 3A; PKG-Iα, protein kinase G-Iα; S654, serine 654.