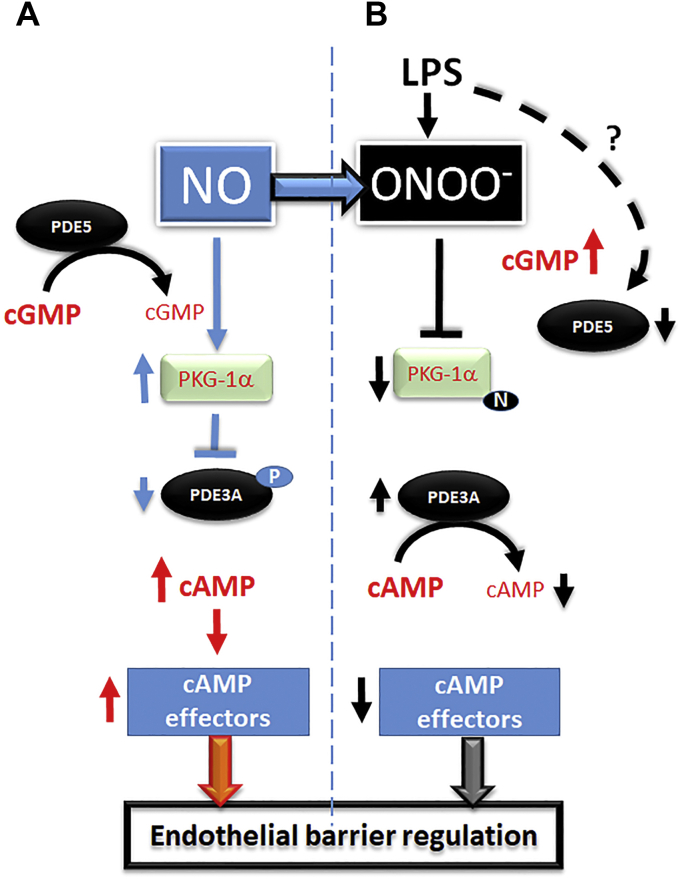
Figure 7.
Schematic representation of PKG-Iα–dependent cAMP/cGMP crosstalk in pulmonary endothelial cells.A, under normal physiological conditions, NO-mediated cGMP generation activates PKG-Iα leading to inhibitory phosphorylation of PDE3A at S654. This results in cAMP elevation and stimulation of barrier-enhancing effectors. B, LPS-mediated generation of peroxynitrite inhibits PKG-Iα via protein tyrosine nitration. Although cGMP levels are high because of LPS-dependent inhibition of PDE5, nitrated PKG-Iα cannot phosphorylate and inhibit PDE3A. This leads to PDE3A-dependent cAMP degradation and impaired barrier function. LPS, lipopolysaccharide; PDE3A, phosphodiesterase 3A; PKG-Iα, protein kinase G-Iα; NO, nitric oxide; S654, serine 654.