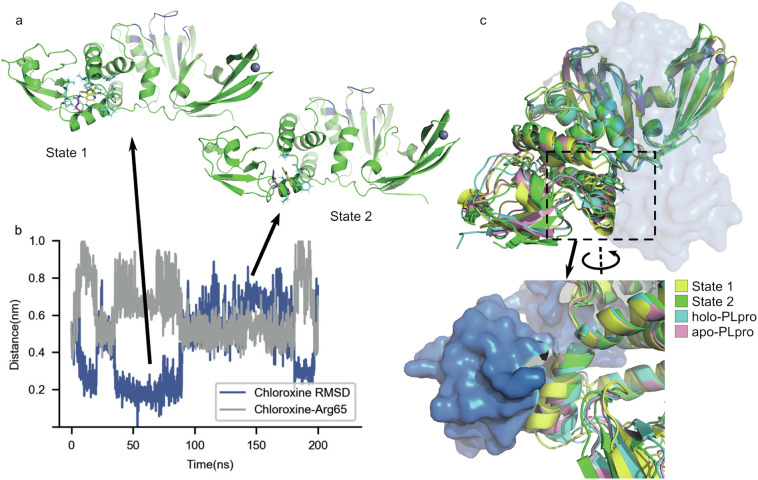
Fig. 4.
Interruption of the PLpro-ISG15 binding interface by binding of chloroxine near Arg65 in a two-state manner. a) The representative conformations of State 1 and State 2 with the same coloring scheme as in Fig. 3a. Arg65 is individually colored magenta. b) The heavy-atom RMSD of chloroxine with State 1 as the reference and the distance between the center of mass of chloroxine and the CZ (the terminal carbon) atom of Arg65 side chain. c) Apo-PLpro, the structures of State 1 and State 2 aligned to the holo-PLpro (PLpro with ISG15 bound) structure. The region of the helix where Arg65 resides is zoomed and rotated 180°for a clearer view. ISG15 in the holo-PLpro structure is colored marine and shown as surface. The structures of apo-PLpro and holo-PLpro were taken from PDB 6XAA[12] and 6YVA [35], respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)