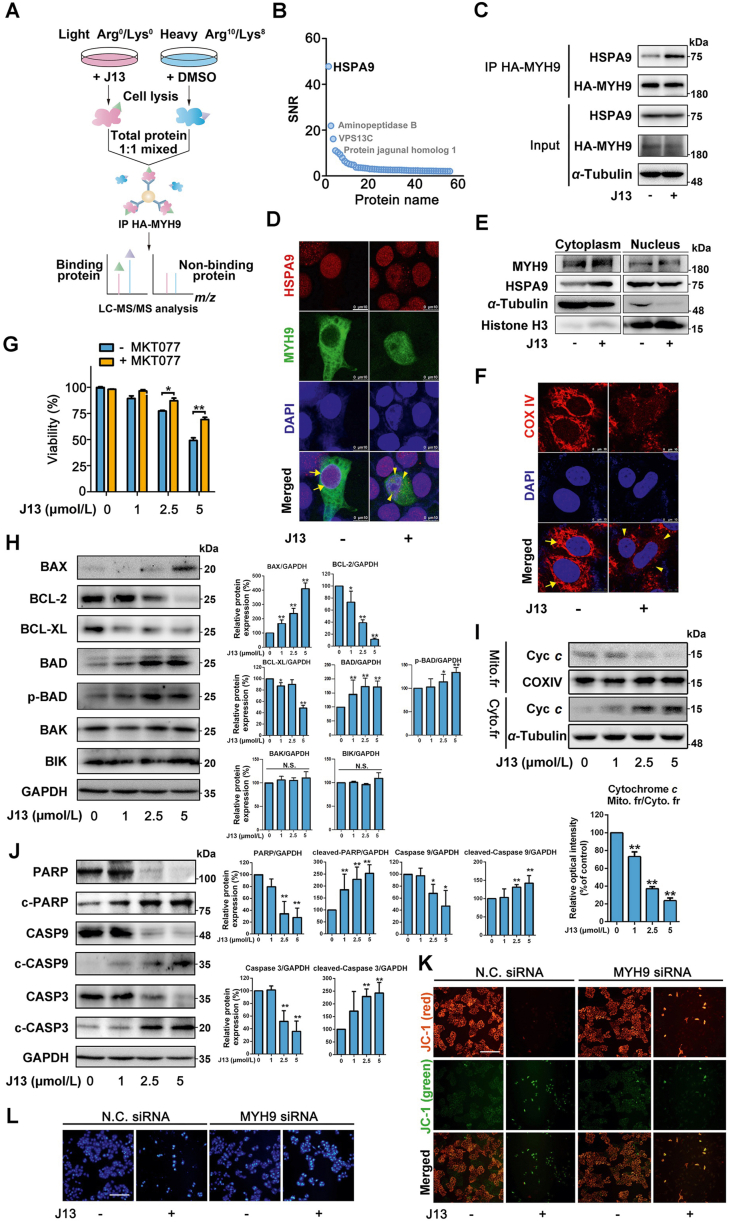
Figure 3.
HSPA9 is a crucial MYH9–actin molecular motor substrate protein for controlling mitochondrial dynamics. (A) Schematic illustration of MYH9-binding proteins identification using pull-down coupled with SILAC analysis. (B) HSPA9 was identified as a binding protein of MYH9. (C) J13 promoted the interaction of HSPA9 with MYH9 by co-IP assay. (D) J13 promoted MYH9 interaction with HSPA9 in nucleus. (E) J13 treatment promoted cytosol translocation of HSPA9, and thus increased co-location of MYH9 with HSPA9. (F) J13 induced mitochondrial fragmentation by disrupting COXIV fluorescence distribution. (G) HSPA9 inhibitor MKT-077 (0.5 μmol/L for 24 h) reversed J13-induced cell viability decrease by MTT assay. (H) J13 significantly induced BAX and p-BAD expressions, and inhibited BCL-2 and BCL-XL expressions. (I) J13 induced cytochrome c translocation from mitochondria to cytoplasm. (J) J13 activated caspase-9/3-dependent apoptosis pathway. (K) MYH9 knock-down reversed J13-dependent mitochondrial depolarization (scale bar = 25 μm). (L) MYH9 knock-down rescued J13-dependent cell apoptosis by Hoechst 33258 staining assay (scale bar = 25 μm). All data are presented as mean ± SD from independent experiments (G: n = 6, H–J: n = 3; ∗P < 0.05, ∗∗P < 0.01.