Abstract
Ginsenosides are a series of glycosylated triterpenoids which belong to protopanaxadiol (PPD)-, protopanaxatriol (PPT)-, ocotillol (OCT)- and oleanane (OA)-type saponins known as active compounds of Panax genus. They are accumulated in plant roots, stems, leaves, and flowers. The content and composition of ginsenosides are varied in different ginseng species, and in different parts of a certain plant. In this review, we summarized the representative saponins structures, their distributions and the contents in nearly 20 Panax species, and updated the biosynthetic pathways of ginsenosides focusing on enzymes responsible for structural diversified ginsenoside biosynthesis. We also emphasized the transcription factors in ginsenoside biosynthesis and non-coding RNAs in the growth of Panax genus plants, and highlighted the current three major biotechnological applications for ginsenosides production. This review covered advances in the past four decades, providing more clues for chemical discrimination and assessment on certain ginseng plants, new perspectives for rational evaluation and utilization of ginseng resource, and potential strategies for production of specific ginsenosides.
Key words: Ginsenoside, Panax species, Biosynthetic pathway, Transcription factors, Non-coding RNAs, Biotechnological approach
Abbreviations: α-AS, α-amyrin synthase; ABA, abscisic acid; ADP, adenosine diphosphate; AtCPR (ATR), Arabidopsis thaliana cytochrome P450 reductase; BARS, baruol synthase; β-AS, β-amyrin synthase; CAS, cycloartenol synthase; CDP, cytidine diphosphate; CPQ, cucurbitadienol synthase; CYP, cytochrome P450; DDS, dammarenediol synthase; DM, dammarenediol-II; DMAPP, dimethylallyl diphosphate; FPP, farnesyl pyrophosphate; FPPS (FPS), farnesyl diphosphate synthase; GDP, guanosine diphosphate; HEJA, 2-hydroxyethyl jasmonate; HMGR, HMG-CoA reductase; IPP, isopentenyl diphosphate; ITS, internal transcribed spacer; JA, jasmonic acid; JA-Ile, (+)-7-iso-jasmonoyl-l-isoleucine; JAR, JA-amino acid synthetase; JAZ, jasmonate ZIM-domain; KcMS, Kandelia candel multifunctional triterpene synthases; LAS, lanosterol synthase; LUP, lupeol synthase; MEP, methylerythritol phosphate; MeJA, methyl jasmonate; MVA, mevalonate; MVD, mevalonate diphosphate decarboxylase; NDP, nucleotide diphosphate; OA, oleanane or oleanic acid; OAS, oleanolic acid synthase; OCT, ocotillol; OSC, oxidosqualene cyclase; PPD, protopanaxadiol; PPDS, PPD synthase; PPT, protopanaxatriol; PPTS, PPT synthase; RNAi, RNA interference; SA, salicylic acid; SE (SQE), squalene epoxidase; SS (SQS), squalene synthase; SPL, squamosa promoter-binding protein-like; SUS, sucrose synthase; TDP, thymine diphosphate; UDP, uridine diphosphate; UGPase, UDP-glucose pyrophosphosphprylase; UGT, UDP-dependent glycosyltransferase; WGD, whole genome duplication
Graphical abstract
Ginsenosides are produced mainly via MVA pathway. Functional genes are regulated by multiple elicitors through different transcriptional factors, which together play pivotal roles in ginsenoside structure diversities.
1. Introduction
Panax genus consists of about 20 species or variants1, 2, 3 (Table 14, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19) among which Panax ginseng C.A. Meyer (Asian or Korean ginseng), Panax quinquefolius L (America ginseng), and Panax notoginseng (Burkill) F.H. Chen (Sanchi ginseng) are especially famous for their wide consumption as food, dietary supplements, functional food and medicinal materials for thousands of years, thus becoming the three best-selling ginseng products20. In addition to the above mentioned valuable species, Panax japonicus C.A. Meyer and its variants21,22, Panax vietnamensis and its variants15,23, and Panax zingiberensis24 are also used as medicinal plants. Ginsenosides (here mean saponins from Panax species) are main bioactive constituents of Panax species, which can be divided into dammarane- and oleanane (OA)-type saponins according to their skeletons. Dammarane-type ginsenosides can be sub-grouped into protopanaxadiol (PPD)-, protopanaxatriol (PPT)- and ocotillol (OCT)-type25 (Fig. 1). Pharmacological researches demonstrated that ginsenosides have multiple bioactivities including anti-inflammation26, 27, 28, anti-cancer29, 30, 31, anti-diabetic32,33, cardiovascular-protective34,35, and others36, 37, 38, 39, 40.
Table 1.
Representative saponins in main root (or rhizome) of Panax species.
| No. | Species | Origin | Representative saponins (content, mg/g) | Group | Ref. |
|---|---|---|---|---|---|
| 1 | P. assamicus Ban. | Sikkim, India | – | – | 4 |
| 2 | P. bipinnatifidus Seem. | Sikkim, India; Nepal | Rb1 (5.2), Rd (1.8), Re (1.5), Rg2 (7.1), Rg1 (2.9), P-RT1 methyl ester, S-R2 methyl ester | III | 5,6 |
| 3 | P. ginseng C.A. Mey. (Asian or Korean ginseng) | Northeast China; Korea | Rb1 (15), mRb1 (10), Rb2 (2), mRb2 (2), Rc (3), mRc (2), Rd (1), Re (6), Rg1 (15), Rf (4) | I | 7 |
| 4 | P. japonicus C.A. Mey. (Chikusetsu-ninjin, Japanese ginseng) | Japan | Ro (107.9–163.0), C-IV (51.8–82.4), C-III (30.4–44.7), C-IVa (4.3–6.7), Re (0–0.7), N-R2 (0.6–1.1) | III | 8 |
| 5 | P. japonicus C.A. Mey. (Satsuma-ninjin, Japanese ginseng) | Southern Kyusyu, Japan | Ro (37.9), C-IV (38.2), C-IVa (2.3), C-III (2.4), Rb1 (3.4), Rc (1.0), Re (2.4), Rg1 (5.0), N-R2 (2.0) | III | 8 |
| 6 | P. japonicus C.A. Mey. (Zhujie Shen, Bai Sanchi) | Yunnan, China | Ro (55.9–79.9), C-IVa (10.9–20.0), C-IV (16.5–33.2), Rg1 (3.6–4.1), Re (2.2–5.3), Rb1 (1.2–3.3), Rd (0.25–0.73), N-R2 (0–0.43), M-R2 (0–6.7) | III | 8 |
| 7 | P. japonicus C.A. Mey. var. major (Burk.) Y. Wu et K.M. Feng (Zhuzi Shen, Daye Sanqi) | Southwest China | Ro (74.1–165.7), C-IVa (22.2–84.2), C-IV (0–1.4), Rd (0.3–11.6), Rb1 (2.1–4.7), Rg1 (0–4.5), Re (0–4.9), N-R2 (0.26–1.0) | III | 8 |
| 8 | P. japonicus C.A. Mey. var. bipinnatifidus (Seem.) C.Y. Wu et K.M. Feng (feather-leaf bamboo ginseng) | Southwest China | Ro (92), Rb1 (2.9), Re (2.5), Rd (0.75), N-R2 (0.32), C-IVa (9.7), C-IV (0.83) | III | 8 |
| 9 | P. japonicus C.A. Mey. var. angustifolius (Burkill) C.Y. Cheng et C.Y. Chu (narrow-leaved Japanese ginseng) | Sichuan, China | C-IV (40.2–45.7), C-IVa (29.4–47.0), Ro (55–81.8), Rb1 (1.4–2.4), Rd (0.4–0.75) | III | 8 |
| 10 | P. notoginseng (Burk.) F.H. Chen (Sanchi ginseng) | Yunnan, China | N-R1 (5.3–7.2), Rg1 (29.6–39.1), Rb1 (26.7–30.6), Rd (5.7–8.4), Re (3.8–5.1), Rc (1.1–1.5) | I | 9,10 |
| 11 | P. pseudoginseng Wall. var. elegantior (Burk.) Hoo et Tseung (pearl pseudoginseng) | China; Nepal; India; Bhutan | Rb1 (7), Re (3), Rg1 (3), Rg2 (0.3), Rd (1), Ro (5), N-R1 (2), N-R2 (0.9), M-R2 (1), P-RT1 (4), P-F11 (0.1), P(S)-F11 (0.6), P-RT2 (0.2), C-IVa (0.4), G-XVII (0.8) (isolated yield from rhizomes) | III | 19 |
| 12 | P. pseudoginseng Wall. subsp. himalaicus (Himalayan ginseng) | Bhutan | Ro (4/72.5), P-RT1 (15/−), C-IVa (17/6), C-IV (−/3), Rb1 (3/10.5), Rd (2/−), Rg1 (4/-), RT3 (1/−) | III | 11,12 |
| 13 | P. quinquefolius L. (American ginseng) | North America | Rb1 (20), mRb1 (18), Re (12), Rg1 (10), Rc (3), mRc (1.5), Rd (2), mRd (2), P-F11 (1.5) | I | 7 |
| 14 | P. sikkimensis Ban. | Sikkim, India | Rb1, Re, Rg1, Rg2, Rb2 (in cell culture) | – | 13 |
| 15 | P. sokpayensis Shiva K. Sharma et Pandit | Sikkim, India | Rb1 (5.2), Rd (7.9), Re (4.4), Rf (0.6), Rg1 (2.4), Rg2 (8.0) | I | 5 |
| 16 | P. stipuleanatus H.T. Tsai et K.M. Feng (Pingbian Sanchi) | Southwest China; North Vietnam | S-R2 (6.3–59.5), S-R1 (1.1–4.6), C-IV (0–4.0) | II | 8,18 |
| 17 | P. trifolius L. (Dwarf ginseng) | North America | Total ginsenosides (Re, Rf, Rg2 and Ro, etc.) content is about 0.06 mg/g | I | 14 |
| 18 | P. vietnamensis Ha et Grushv. (Vietnamese ginseng) | Southwest China; North Vietnam | M-R1 (7.2), M-R2 (93.5), P-RT4 (2.4), V-R11 (8.7), V-R1+V-R2 (33.7), Rb1 (8.1), Rb2 (3.6), Rd (2.3), Rg1 (10.9), Re (1.1), N-R1 (3.5), N-R2 (3.0) | I | 15 |
| 19 | P. vietnamensis var. fuscidiscus | Southwest China; North Vietnam | M-R2 (57.8–84.8), Rg1 (23.1–56.7), N-R1 (7.6), N-R2 (0.7–1.7), Re (1.0–3.7), Rb1 (6.2–15.6), Rb2 (3.9), Rc (0.8–2.3), Rd (0.2–6.0) | I | 8 |
| 20 | P. vietnamensis var. langbianensis | Vietnam | – | – | 3 |
| 21 | P. wangianus S.C. Sun | Meghalaya, India | – | – | 16 |
| 22 | P. zingiberensis C.Y. Wu et K.M. Feng (ginger ginseng) | Southwest China; North Vietnam | Ro (68.3–93.9), C-IV (27.3–38.5), C-IVa (7.8–8.0), Rg1 (20.5–23.8), Rb1 (1.4–3.4), Rc (0.3–0.9), N-R2 (0–0.2), Z-R1 (0.8) | III | 8,17 |
Group I plants mainly contain dammarane-type ginsenosides; Group II plants mainly contain OA-type saponins; Group III plants contain plenty of both dammarane-type and OA-type saponins. Saponin content is obtained from one of the cited literatures as a reference, and it varies due to different growing areas and ages of plants as well as sample preparation and analytical method. The saponin contents of P. pseudoginseng Wall. subsp. himalaicus and P. japonicus C.A. Mey. growing in different places are quite distinct from each other as descripted in literatures. C-: chikusetsusaponin, G-: gypenoside, M-: majonoside, N-: notoginsenoside, P-: 24(R)-pseudoginsenoside, P(S)-: 24(S)-pseudoginsenoside, S-: stipuleanoside, V-: vinaginsenoside R1, Z-: zingibroside.
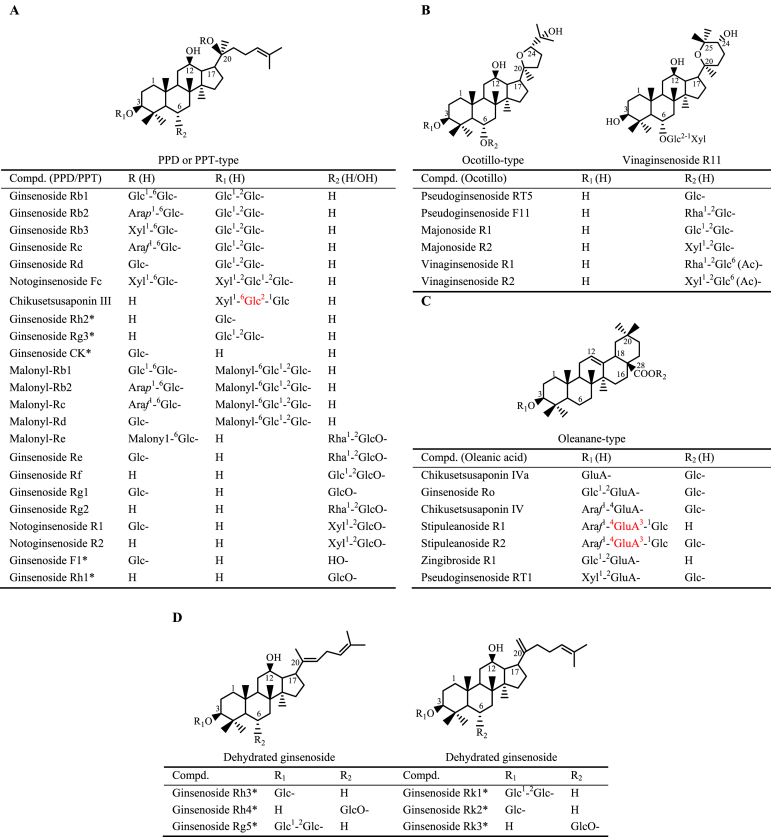
Figure 1.
Representative and hot ginsenosides in Panax species. (A) PPD or PPT-type ginsenosides. (B) Ocotillo-type ginsenosides. (C) Oleanane-type ginsenosides. (D) Dehydrated ginsenosides. Ginsenoside metabolites (hot compounds) are marked by asterisk. Chikusetsusaponin III, stipuleanoside R1 and R2 are branched glycosides, sapogenins link to C-1 of Glc or GluA (marked by red).
Till 2013, the global market of ginseng products is estimated to be more than USD 2000 million with P. ginseng taking the largest proportion, followed by Panax quinuefolius, P. notoginseng, and P. japonicus20. In 2016, fresh ginseng output in China was 28,900 tons, leading to about USD 7500 million value in Jilin ginseng industry41. As the demand for ginseng products increases, ginseng cultivation and alternative approaches to produce ginseng products have become hot research topics42. Hence, a thorough understanding of the structures, distributions, and biosynthetic pathways of ginsenosides is important for quality evaluation of herbal medicines, rational utilization of natural resource and production of ginsenosides by biotechnology.
There are excellent reviews available including those concerning ginsenosides focusing on structures or their bioactivities43,44, on isolation and analysis45, 46, 47, on special ginsenoside such as OCT-type saponins and saponin stereoisomers48,49, on metabolic regulation50, 51, 52, 53, and on elicitation strategy and signal transductions54. In contrast, reviews on ginsenoside biosynthesis are limited and mainly focusing on sapogenin biosynthetic genes and their applications55.
In this review, we summarized the representative saponins of every known Panax species, reviewed the biosynthetic pathways from the formation of both the sapogenin and the sugar donor aspects, highlighted the enzymatic processes leading to ginsenoside structure diversities, and provided new insights for understanding the biological origin of diversified ginsenosides. We discussed the topic in four main sections: (1) the distribution of representative ginsenosides from nearly 20 Panax species including species-specific ginsenosides, malonylated ginsenosides, and minor ginsenosides, (2) the pathways of saponin biosynthesis including enzymes in saponin skeleton biosynthesis, in UDP-sugar formation, and in skeleton decoration, emphasizing OSCs, CYPs, and UGTs that responsible for saponin structure diversities, (3) transcription factors (TFs) in ginsenoside biosynthesis and non-coding RNAs (ncRNAs) regulating ginseng plant growth, and (4) the advanced efforts on biotechnological approaches for the production of ginsenosides in recent years.
2. Diverse ginsenosides in Panax species as natural resources
About 300 ginsenosides have been isolated and identified from different Panax species25. Some of them are important as chemical markers for their distinctive distributions, parts of them are attracting as natural resources for their high content in plants, and a few of them are becoming hot molecules for their particular bioactivities. It is of great significance to summarize representative saponins of each Panax species, which will benefit for the clarification of their divergent biosynthesis pathways to provide biotechnological approaches and produce the hot compounds.
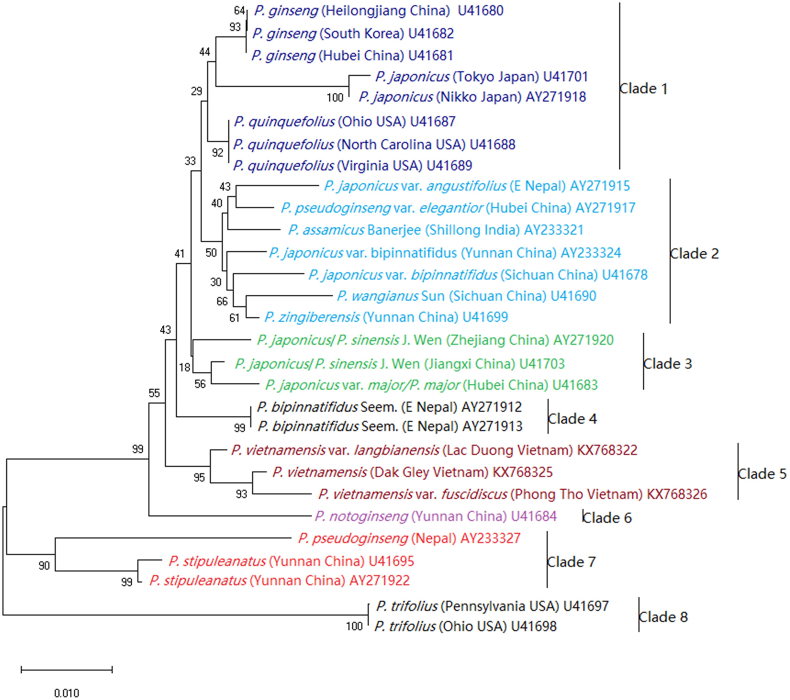
About 20 Panax species, distinctly growing in the eastern North America and East Asia, have been reported1, 2, 3. Phylogenic analysis with internal transcribed spacer (ITS)56, chloroplast DNA (cpDNA) restriction site and ITS57, trnC-trnD1, or trnK and 18S rRNA sequence58 all showed the diversities and complexes of the East Asia species. Zuo et al.59 later classified them as seven well-divergent species (Panax trifolius, Panax pseudoginseng, Panax stipuleanatus, P. notoginseng, P. ginseng, P. quinquefolius, and P. japonicus C.A. Mey.) and a not well-defined group named Panax bipinnatifidus species complex using DNA barcode. The P. bipinnatifidus species complex that was mainly located in Sino-Himalayan region and was previously treated as part of P. pseudoginseng56, was separated into 10 groups using amplified fragment length polymorphism (AFLP) makers60, and P. zingiberensis, P. vietnamensis, Panax wangianus and P. bipinnatifidus Clades split from this species complex as analyzed by restriction-site associated DNA sequencing (RAD-seq) method61. East Asia was the diversification centers of Panax species, and southeastern Yunnan of China might have acted as the corridor for Panax species genetic exchanges and dispersals62. During the dispersal and evolution process, P. ginseng, P. quinquefolius, and P. japonicus (from Japan) become tetraploid (P. zingiberensis may also be a tetraploid) by whole genome duplication (WGD) events to adapt the cooler environments, while most of the other species are diploid61, 62, 63. A phylogenetic tree of Panax plants based on widely used ITS gene3,56,57 were constructed here to better understand their evolution relationships (Fig. 2). As displayed, the 29 ginseng samples were clustered into 8 Clades (or Subclades).
Figure 2.
Phylogenetic tree of Panax plants based on ITS gene by Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The ID of ITS in GenBank are shown in the tree. P. japonicus from China was also treated as P. sinensis by Jun Wen et al.56.
Saponins in plants of Clades 1–3 were OA- or dammarane-type, while saponins in species of Clades 5 and 6 were mainly dammarane-type. Their content was varying from 0.06 mg/g in P. trifolius of Clade 8, 5.8–15.6 mg/g in P. ginseng of Clade 1, 55.0–70.4 mg/g in P. notoginseng of Clade 6, 68.1–167.1 mg/g in P. vietnamensis of Clade 5 to 192.8–296.2 mg/g in P. japonicus of Clade 1 (Table 1). Base on chemical profiles, Panax genus could be divided into three groups, although they were classified into two groups previously8. P. ginseng7, P. notoginseng10, P. quinquefolius7, and P. vietnamensis and its varieties15,22 were classified in Group I characterized by high amounts of dammarane-type ginsenosides. P. stipuleanatus was a Group II species for having high content of OA-type ginsenosides only8. Group III species included P. zingiberensis8, P. bipinnatifidus Seem.5,6,64, P. japonicus and its varieties8 for containing both large amount of OA-type ginsenosides and dammarane-type ginsenosides. The grouping of other ginseng species and their reported representative saponins were listed in Table 1.
2.1. Representative ginsenosides
The composition of ginsenosides varies within the same species grown in different area. For example, P. japonicus collected from China (named Zhujieshen) and Japan (named Chikusetsu-ninjin) are distinct in ginsenosides composition. Although both of them had plenty of OA-type Ro (55.9–163.0 mg/g), C-IV (16.5–82.4 mg/g) and C-IVa (4.3–20.0 mg/g), the latter was rich in PPD-type chikusetsusaponin III (C-III) (30.4–44.7 mg/g) but the former did not have this compound8. The former also had a considerable amount of Rg1, Re, Rb1 and Rd, while the latter only had minor of Re, N-R2, Rg1 and Rb18 (Table 1). Satsuma-ninjin, a special population of P. japonicus in southern Kyusyu Japan, which had plenty of Ro (37.9 mg/g), C-IV (38.2 mg/g), and considerable amount of C-IVa, C-III, Rb1, Rc, Re, Rg1 and N-R28 (Table 1), is also distinct to P. japonicus in China. C-III is a marker constituent in P. japonicus from Japan, present in both Chikusetsu-ninjin and Satsuma-ninjin8.
In addition, the content and the ratios of individual ginsenosides are varied in different parts within a species. For instance, the total saponin content in the rhizomes, main roots, fine roots, leaves, and stems of P. ginseng was about 142.4, 63, 142.5, 92 and 8.6 mg/g, respectively7. The most abundant ginsenosides were Rb1, Rg1, malonylginsenoside Rb1 (mRb1), Re, Rf, Rb2, mRb2, Rc and mRc in the main roots (their contents ranging from 2 to 15 mg/g, Table 1), Re (22 mg/g), Rg1 (10 mg/g), Rd (15 mg/g), mRd (12 mg/g), Rb2 (6 mg/g), mRb2 (4.5 mg/g), Rc (6 mg/g) and mRc (3 mg/g) in leaves, and Re (3 mg/g), Rg1 (2 mg/g) and mRd (1 mg/g) in stems, respectively7. Ginsenoside Rb1 and mRb1, the two most plentiful saponins in underground parts of P. ginseng, could not be quantified in some aerial parts due to the low concentrations7. As for P. quinquefolius, the total saponin content in fine roots, main roots, rhizomes, stems, and leaves was about 116, 76, 107, 13 and 37 mg/g, respectively. The major ginsenosides in leaves of P. quinquefolius were ginsenosides Re (10 mg/g), P-F11 (6 mg/g), mRb2 (4 mg/g), Rg1 (3 mg/g), Rb3 (2–3 mg/g) and mRd (2 mg/g), while those in its main roots were Rb1, mRb1, Re, Rg1, Rc, mRc, Rd, mRd, and P-F11 (1.5–20 mg/g, Table 1)7. Regarding to P. notoginseng, the content of total ginsenosides in the main roots, rhizomes, stems, and leaves was about 75.7–89.8, 137.5, 10.8 and 109.2 mg/g, respectively9. The leaves had high content of PPD-type saponins including ginsenosides Rb3 (25.4–32.6 mg/g), Rc (14.0–16.3 mg/g), Rb2 (4.8–6.7 mg/g), notoginsenoside Fc (N-Fc, 8.2–13.4 mg/g), and traceable N-Fe (0–0.58 mg/g) and N-Fd (0–0.98 mg/g), but no PPT-type ginsenosides65, whereas the main roots were rich in both PPT-type ginsenosides Rg1, Re and N-R1, and PPD-type ginsenosides Rb1, Rd and Rc9 (1.1–39.1 mg/g, Table 1). Ginsenoside Rf, P-F11 and N-R1 were regarded as distinctive compounds in P. ginseng, P. quinquefolius and P. notoginseng, respectively66. Malonylginsenosides, such as mRc and mRb2, were also considered as differential compounds between P. ginseng and Panax notginseng66. In P. vietnamensis, total saponin content was about 195.2, 155.9 and 139.3 mg/g in rhizomes, radices, and fine roots, respectively, with majonoside R2 (M-R2), Rg1, Rb1, vinaginsenoside R11 (V-R11), V-R1, V-R2, and M-R1 being the richest15. M-R2, the characteristic and the most abundant saponin in roots of P. vietnamensis, was not found in its leaves15.
Traditionally, rhizomes and roots of ginseng species are used for medicinal therapy and healthcare. Leaves of P. ginseng, P. notoginseng, and P. quinquefolius are all good source of specific ginsenosides that attract researchers to explore as alternative medicinal material resources. As for most of the other Panax species, ginsenosides compositions in their aerial parts still require to be explored.
2.2. Malonylginsenosides
Malonylginsenosides, a kind of acidic ginsenosides, comprise a large proportion of saponins in Panax genus67, 68, 69. The ratio of the total content of neutral ginsenosides (unmalonylated ginsenosides herein, Rb1, Rb2, Rb3, Rc, Rd, Rg1 and Re) to the corresponding malonylginsenosides in flowers of P. notoginseng, P. ginseng and P. quinquefolius were 5.52 ± 1.33%, 3.2 ± 0.64% and 2.39 ± 0.57%, respectively69. The content of mRb1 in the roots of P. ginseng and P. quinquefolius was up to 10 and 18 mg/g, respectively7. Recently, more than 100 malonylginsenosides have been tentatively characterized from P. ginseng, P. quinquefolius and P. notoginseng68. About 20 malonylginsenosides have been separated and identified from roots and flower buds of P. ginseng67,70. Malonyl groups were found linking to different positions of the first or the second glucose residues in C-3/C-20 of PPD-type and C-6/C-20 of PPT-type malonylginsenosides, some of which had potential antidiabetic activities67.
2.3. Minor ginsenosides
Minor ginsenosides usually refer to saponins that naturally occur at low concentrations (less than 0.1%) in Panax species with the most important being ginsenoside artifacts. The artifacts are mainly deglycosylated or dehydrated products or 20(R)-epimers. Some of them are hot molecules due to the high value as medicinal and healthcare materials. Major ginsenosides can be converted to minor ones through the drying and steaming process, and thus red ginseng is enriched in minor ginsenosides as it is made through these processes71. Microbiota-mediated metabolites of ginsenosides is another source of minor ones72. Ginsenosides Rg3 and Rh2 are two minor ginsenosides known for their anticancer activities30,31. Ginsenoside compound K (CK) is another minor saponin which has been paid much attention for its multiple bioactivities73,74. Ginsenosides Rg5 and Rk1, both of which showed potential anticancer activities75,76, are two dehydration products of ginsenoside Rg3, and are enriched in steamed ginseng products77. The 20(R)-epimers of both Rg3 and Rh2 have also gained wide attention for their bioactivities78,79. Our group have revealed that notoginsenoside Ft1, a rare 20(R)-epimer of PPD-type saponin, has potential hemostatic, pro-apoptotic, angiogenesis and other activities80, 81, 82. Minor PPT-type ginsenoside Rh1, which content is increased in red ginseng83, has antioxidant, anti-inflammatory, and immunomodulatory effects84.
3. Biosynthetic pathways of ginsenosides
As mentioned above, ginsenosides are distinguished from other saponins by dammarane- or OA-type triterpene scaffolds decorated with one or more sugar chains. Correspondingly, the biosynthesis of ginsenosides contains three main processes, the formation of ginsenoside skeletons, the synthesis of sugar donors, and the skeletons modification processes.
3.1. Ginsenoside skeleton formation
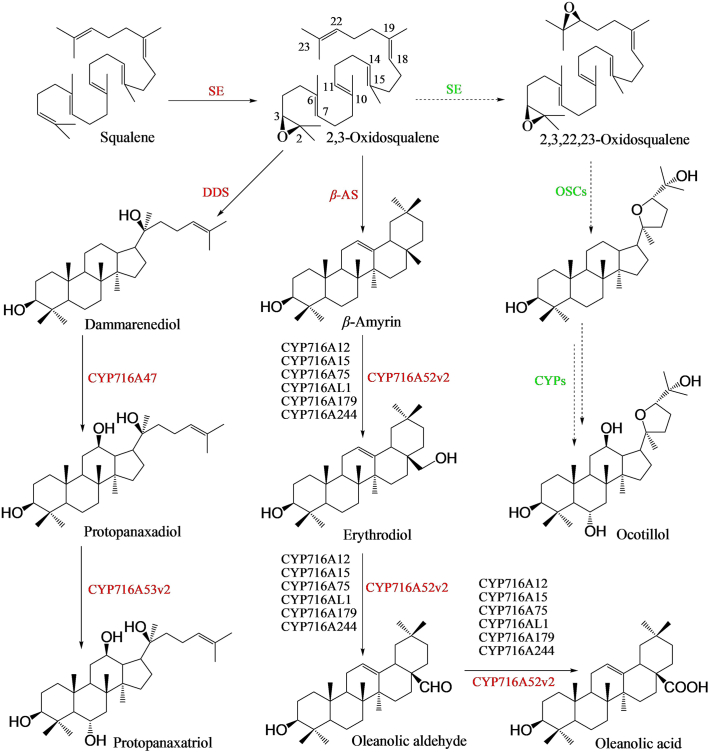
(3S)-2,3-Oxidosqualene, the mono-oxidative product of squalene by SE85, is the key precursor for the biosynthesis of both dammarane-type ginsenosides via DM cyclized by dammarenediol-II synthase (DDS) and OA-type ginsenosides via β-amyrin catalyzed by β-amyrin synthase (β-AS)86,87 (Fig. 3). Squalene comes from the condensation reaction of isopentenyl diphosphate (IPP or IDP) and dimethylallyl diphosphate (DMAPP or DMADP) which are originated from either the cytosol MVA pathway or the plastid MEP pathway88,89. The modified MVA pathway discovered in archaea may also contribute to the formation of IPP through phosphomevalonate decarboxylase (MVAPD) and isopentenyl phosphate kinase (IPK) catalyzing reactions instead of phosphomevalonate kinase (PMK) and mevalonate diphosphate decarboxylase (MVD)90,91.
Figure 3.
CYPs in the biosynthesis of different ginsenoside skeletons. CYPs marked in red and dark are identified from Panax species and other species, respectively. Solid arrows are proven pathways, dashed arrows are unproven pathways, and double arrows are multiple steps (similarly hereinafter). The abbreviations are indicated as followed, β-AS: β-amyrin synthase, CYP (s): cytochrome P450 (s), DDS: dammarenediol synthase, OSC (s): oxidosqualene cyclase (s), SE: squalene epoxidase.
Both MVA pathway and MEP pathway are involved in ginsenoside biosynthesis, and MVA pathway plays a more important role89. HMGR was considered as a rate limiting enzyme and was always selected as regulatory target in MVA pathway92. Overexpression of HMGR enhanced the production of ginsenoside in P. ginseng and P. notoginseng92,93. While MVD, another enzyme in MVA pathway played a key role in phytosterol rather than ginsenoside biosynthesis in P. ginseng94.
3.1.1. Squalene biosynthesis enzymes
Squalene is synthesized from two FPP molecules by SS, while FPP is condensed by FPPS that catalyzes the sequential addition of two molecules of IPP to DMAPP to form first geranyl diphosphate (GPP) and then FPP. PnFPPS was highly expressed in flowers, leaves and stems of four years old P. notoginseng, but less in roots95. Expression patterns showed that the total saponins accumulation might result firstly from the contribution of FPS, then from SS and DS96. Overexpression of PgFPPS in P. ginseng hairy roots led to total ginsenosides increasing to approximately 2.4-fold of the control94.
Three SS namely, PgSS1, PgSS2, and PgSS3, were functionally characterized from P. ginseng. PgSS1 (mRNA) was highly expressed in all organs, whereas PgSS2 and PgSS3 were only found in specific tissues97. Co-overexpression of PnSS and PnHMGR in P. notoginseng cells, resulted in total ginsenosides increasing to 3- and 1.5-fold of the control and the cell line overexpressed PnHMGR alone, respectively92.
3.1.2. Squalene epoxidases (SEs)
SE catalyzes the oxygenation of squalene to (3S)-2,3-oxidosqualene, and is one of the rate-limiting enzymes in this pathway (Fig. 3). Four to twelve SE isoforms were predicted in P. ginseng98,99 and five isoforms were in P. notoginseng100. PnSE1 and PnSE2, belonging to two separate large groups, were similar to PgSE1 and PgSE2, respectively95. Phylogenetic analysis showed that the SEs from P. ginseng, P. vietnamensis, and P. notoginseng formed a clade, while the SE of Panax quinquefolium clustered outside101, possibly due to the incompletely identification of multiple divergent SE homologs in P. quinquefolium. In situ hybridization experiments indicated that both PgSE1 and PgSE2 accumulated preferentially in vascular bundle tissue and resin ducts of petioles. RNAi of PgSE1 completely suppressed PgSE1 transcription but strongly upregulated PgSE2 and PNX (cycloartenol synthase gene) mRNA, leading to the reduction of ginsenosides but the enhancement of phytosterols85.
3.1.3. Oxidosqualene cyclases (OSCs)
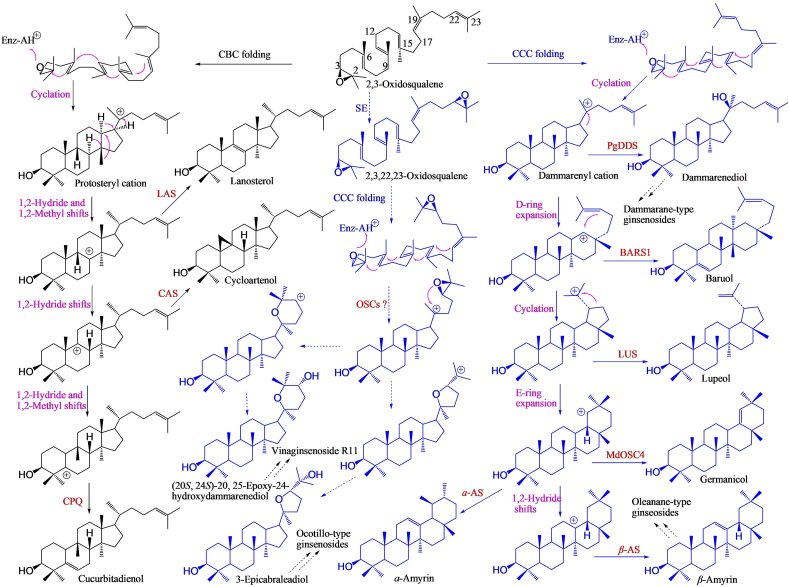
The OSCs-catalyzed cyclization of (3S)-2,3-oxidosqualene is the first committed step in the biosynthesis of diverse triterpenoid saponins and phytosterols102. To date, more than 80 functionally different OSCs have been characterized in plants, contributing to the formation of diverse triterpenoid and steroid skeletons103,104. There are about 13–19 genetically different OSCs in P. ginseng genome, encoding lanosterol synthase (LAS), cycloartenol synthase (CAS), DDS and β-AS98,99. DDS cyclizes (3S)-2,3-oxidosqualene to DM, which can be further oxidized to PPD and PPT. Several isoforms of DDS have been characterized including PgDDS from P. ginseng105, PqDDS from P. quinquefolius106, PnDDS from P. notoginseng95,101 and CaDDS from Centella asiatica107. DDS within Panax species shows about 97% identity with each other101, while CaDDS from C. asiatica shows about 78% identity with DDS from Panax species. β-AS from P. ginseng (PNY1 and PNY2), which catalyzes the formation of β-amyrin, has 56% identity with PgDDS108. The OSCs catalyzing the formation of the skeleton of M-R2, a major ginsenoside in P. vietnamensis15, has not been reported yet. It was deduced that such OSCs might use (3S,22S)-2,3,22,23-dioxidosqualene as substrate to generate 3-epicabraleadiol (Fig. 4). This speculation was supported by the result that incubating (3S,22S)-2,3,22,23-dioxidosqualene with Arabidopsis lupeol synthase 1 (AtLUP1) expressed by yeast could produce epoxydammaranes and olefinic analogues, one of which was 3-epicabraleadiol, the precursor of ocotillol109. V-R11 with the content being about 8.7 mg/g in P. vietnamensis rhizome15, has a similar skeleton with M-R2. It might also generate from (3S,22S)-2,3,22,23-dioxidosqualene but via (20S,24S)-20,25-epoxy-24-hydroxy-dammarenediol by the same or similar OSC with that of M-R2.
Figure 4.
Comparison of different cyclization mechanisms among DDS, β-AS and other OSCs. Products labeled in black are generated via protosteryl cation, while products labeled in green are synthesized via dammarenyl cation. The abbreviations are indicated as follows, α-AS: α-amyrin synthase, β-AS: β-amyrin synthase, BARS1: baruol synthase, CAS: cycloartenol synthase, CCC: chair-chair-chair, CBC: chair-boat-chair, CPQ: cucurbitadienol synthase, LAS: lanosterol synthase, LUP: lupeol synthase, MdOSC4: oxidosqualene cyclase 4 of Malus domestica, PgDDS: dammarenediol synthase of P. ginseng.
OSCs can be divided into two groups based on the different reaction mechanisms, the protosteryl cation mechanism and the dammarenyl cation mechanism103,104. The protosteryl cation serves as an intermediate leading to tetracyclic cycloartenol, lanosterol and cucurbitadienol via chair-boat-chair (CBC) conformation. The dammarenyl cation makes tetracyclic dammarenediol-II and baruol, or pentacyclic lupeol, β-amyrin, α-amyrin, germanicol and a series of other uncommon triterpenes via chair-chair-chair (CCC) conformation110,111. The cyclization mechanisms from oxidosqualene to triterpenoids and phytosterols are summarized in several articles104,112. Briefly, the cyclization involves four steps: 1) substrate binding and folding, 2) reaction initiation by protonation of the 2,3-epoxide, 3) cyclization, sometimes ring expansion and (or) skeleton rearrangement by 1,2-methyl and (or) -hydride shifts, and 4) termination by deprotonation or hydrated reactions. Here, we compared the different cyclization mechanisms of DDS, β-AS and other OSCs to fully understand the relationships between them (Fig. 4).
DDSs are uncommon in nature, which result in the unique ginsenoside scaffolds predominant in Panax species, and β-ASs are widespread in plant kingdom such as in Araliaceae113, Umbelliferae114, Leguminosae115 and other families116,117. DDS and β-AS from Panax genus are single product producing enzymes, while some other OSCs are multifunctional enzymes. For example, CrAS from Catharanthus roseus generated α- and β-amyrin in a 5:1 ratio116, and MdOSC4 from Malus domestica catalyzed the formation of germanicol, β-amyrin, and lupeol in the proportion of 82:14:4110. In addition, a single mutation enabled lupeol synthase (LUP) had the function of β-AS118. All these results indicated that β-AS, α-AS and LUP were genetically and functionally closely related with each other.
The function of DDS and β-AS has also been investigated in vivo. Silencing of DDS expression in transgenic P. ginseng resulted in a reduction of ginsenoside production to 84.5%, indicating that DDS played a vital role in ginsenoside biosynthesis108. Transgenic P. notoginseng cells with β-AS from P. japonicus (Pjβ-AS) produced C-IV and C-IVa, two OA-type saponins119, and transgenic Pjβ-AS rice produced OA-type sapogenin, demonstrating that β-AS was engaged in the biosynthesis of the precursor of OA-type ginsenosides87. Suppressing β-AS, PNY1 and PNY2 in P. ginseng hairy root resulted in the obvious decrease of OA-type ginsenoside (Ro) but the increase of dammarane-type ginsenosides (Rb1 and Rg1) and total ginsenosides120.
3.2. Cytochrome P450s (CYPs)
Cytochrome P450s (CYPs) are heme-containing oxygenases with primary monooxygenase activity that introduce an oxygen atom from oxygen molecules into hydrophobic substrates generating more hydro-soluble products121. The oxygen atoms introduced by CYPs provide anchoring points for further decorations, significantly expanding the skeleton diversity122, which is another essential process for the structural diversities of ginsenosides.
In plants, CYPs constitute one of the largest family of enzymatic proteins123, but the reported CYPs related to the formation of ginsenosides are limited. Three CYPs, CYP716A47 (PPDS), CYP716A53v2 (PPTS) and CYP716A52v2 (OAS), have been identified from P. ginseng through homology-based cloning and in vivo or in vitro activity screening (Fig. 3)124, 125, 126. CYP716A47 could oxidize C-12 of DM to produce PPD which was further hydroxylated at C-6 by CYP716A53v2 to generate PPT. Different from the monooxygenase activity of CYP716A47 and CYP716A53v2, CYP716A52v2 was a multifunctional oxygenase participating in the oleanolic acid biosynthesis. It oxidized β-amyrin at the C-28 position to form oleanolic acid via erythrodiol intermediate through three-step reactions (Fig. 3)127, 128, 129, 130. Six other CYPs, with 70% similarity to CYP716A52v2 including MtCYP716A12 from Medicago truncatula128, VvCYP716A15 from Vitis vinifera127, GuCYP716A179 from Glycyrrhiza uralensis130, CrCYP716AL1 from C. roseus131, EsCYP716A244 from Eleutherococcus senticosus113, and MlCYP716A75 from Maesa lanceolata129, had the same activities with CYP716A52v2, but the first four enzymes could also catalyze the oxidation of C-28 of α-amyrin and lupeol127,130,131. All the three characterized CYPs in P. ginseng belong to CYP716A subfamily, yet their functions are quite different from each other and they diversified ginsenoside structures further.
3.3. Biosynthesis of UDP-sugars
Sugar moieties are highly related with the biological activities of ginsenosides. Nucleoside diphosphate (NDP)-sugars were used as sugar-donors for glycosides biosynthesis, and UDP-sugars are the preferred ones for the glycosylation of secondary metabolites in plants132,133. Various sugar donors contribute to ginsenoside structure diversities leading to function and bioactivity diversified ginsenosides. UDP-α-d-glucose (UDP-Glc) was mostly used in ginsenoside biosynthesis, and β-d-glucosidic linkages were found almost in every ginsenosides134,135 (Fig. 1). Apart from UDP-Glc, other sugar donors are also participating in the ginsenoside skeleton modifications such as UDP-GluA, UDP-Xyl, UDP-Gal, UDP-Arap, UDP-Araf and UDP-Rha.
Biosynthetic pathways for these UDP-sugars are diverse with the “salvage” and the interconversion pathways being the two main routes in plants133. Sucrose synthase (SUS) and UDP-glucose pyrophosphosphprylase (UGPase) are the two key enzymes respectively utilizing sucrose and α-d-glucose-1-phosphate (G1P) as the direct precursors in UDP-Glc biosynthesis to produce UDP-Glc133. The biosynthesis of UDP-sugars has not been explored in Panax species, let alone which route has the highest metabolic flux efficiency to produce specific ginsenosides. The conservative pathways of UDP-sugars in other plants will provide a reference for the study in ginseng species.
3.4. UDP-dependent glycosyltransferases (UGTs)
The glycosylation of most ginsenoside triterpene scaffolds is catalyzed by UGTs using UDP-sugars mentioned above as sugar donors to form diverse ginsenosides. Ginsenoside sapogenins are further decorated at C-3 or/and C-20 hydroxyl groups of PPD-type, C-6 or/and C-20 hydroxyl groups of PPT-type and C-3 hydroxyl or/and C-28 carboxyl groups of OA-type saponins. This is the third step for structural diversities of ginsenosides.
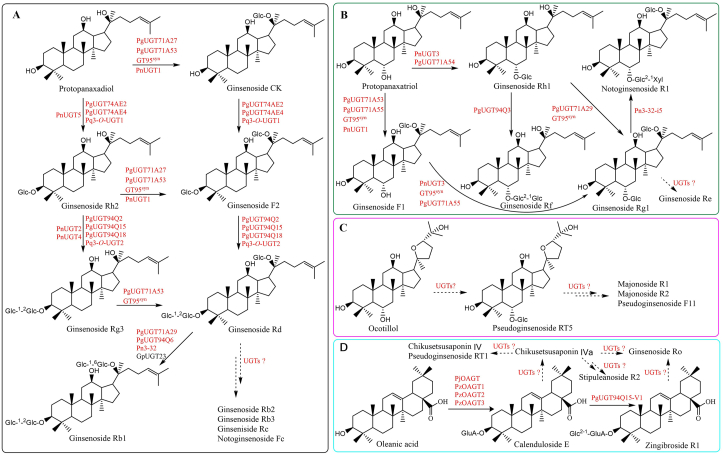
The nomenclature of UGTs is based on the homology of their amino acid sequences. A sequence homology >40% defines the family, while a homology >60% defines the subfamily136. Plant UGTs belonging to superfamily 1 GTs are assigned to the UGT family 71–100. The superfamily 1 GTs exhibit inverting catalytic mechanism and depict GT-B fold which contains two Rossmann folds in the N- and C-terminal residues, respectively134,135,137. The ginseng genome encodes a large diverse set of UGTs. About 225 or 226 UGTs have been predicted, accounting for one of the largest gene families in ginseng98,99. The encoding enzymes were assigned to 24 subfamilies, with UGT73 being the most abundant family, followed by UGT74 and UGT9498. A total of 158 UGTs encoding predominate UGT71, UGT73, UGT74, UGT85, UGT91 and UGT94 subfamilies were predicted in genome of P. notoginseng, and five of them, PnUGT1–5, were functionally characterized100. To date, dozens of UGTs that participate in the biosynthesis of dammarane-type saponins have been identified from Panax species (Fig. 5). These UGTs belonged to UGT71, UGT74 or UGT94 families. UGTPg1 (PgUGT71A53) catalyzed the glycosylation of the hydroxyl group at the C20 position in PPD and PPT to produce compound K and ginsenoside F1, respectively138, 139, 140. UGTPg100 (PgUGT71A54) specifically glycosylated the C6-OH of PPT to produce ginsenoside Rh1139,140. While UGTPg101 (PgUGT71A55) could glycosylate both C20-OH of PPT and C6-OH of F1 to generate ginsenoside F1 and Rg1, respectively139,140. PgUGT74AE2 and two of its homologs, UGTPg45 (PgUGT74AE4) and Pq3-O-UGT1, catalyzed the glycosylation of the C3-OH of PPD to produce ginsenoside Rh2. UGTPg29 (PgUGT94Q2) and its homologs Pq3-O-UGT2 could transfer glucose onto the C-2ʹ hydroxyl group of the first glucose residue at C-3 of ginsenoside Rh2 to produce ginsenoside Rg3140, 141, 142, 143, 144, which were proved by over expression and RNAi experiments concerning Pq3-O-UGT1, Pq3-O-UGT2 and PgUGT94Q2 in transgenic P. quinquefolius and P. ginseng hairy roots143,144. UGT71A29 has high similarity with UGTPg1 at amino acid level (96.47%). It could glycosylate Rh1 and Rd to produce ginsenosides Rg1 and Rb1, respectively145. Both Pn3-32 from P. notoginseng and GpUGT23 from Gynostemma pentaphyllum belong to UGT94 family. Both of them have low identity with UGT71A29 (less than 30%) but could also convert ginsenoside Rd to Rb1, and Pn3-32 could also catalyze F1 to form Rg1135,146. Interestingly, Pn3-32-i5, another UGT94 family member from P. notoginseng, could convert ginsenoside Rg1 to notoginsenoside R1 by adding a xylose moiety thus was identified as UDP-Xylose transferase, although it has >90% identity with Pn3-32 and PgUGT94Q2146. A serial of UGTs in UGT94 family with high amino acid identity, ranging from 85.87% to 99.78%, from P. ginseng and P. notoginseng have been cloned and characterized, recently140. Most of them had the activity of catalyzing Rh2 to produce Rg3 (PgUGT94Q18 showed the highest activities), some of them could extend the sugar chain at C3-O-Glc and C20-O-Glc of PPD-type saponins, or C6-O-Glc and C20-O-Glc of PPT-type saponins. Particularly, PgUGT94Q15-V1 could lengthen the sugar chain at C3-O-Glc of calunduloside E to produce zingibroside R1, an OA-type saponin discovered in P. zingiberensis140. Our team found that GT95syn (belong to UGT71 family) originated from P. notoginseng, could transform 20(R)-PPD and 20(R)-PPT to produce 20(R)-CK and 20(R)-F1, respectively147. GTK1 and GTC1 from Bacillus subtilis were able to glycosylate the C3-OH of 20(R)-PPD to form 20(R)-Rh2147. The functional elucidated UGTs provided useful tools for production of diversified ginsenosides including those (R)- and (S)-isomers in the future.
Figure 5.
UGTs catalyzing ginsenoside biosynthesis identified from Panax species (A)−(D) show UGTs involved in PPD-, PPT-, OCT- and OA-type ginsenoside biosynthesis, respectively. The abbreviations are indicated as follows, Pg: P. ginseng, Pj: P. japonicus var. major, Pn: P. notginseng, Pq: P. quinquefolius, Pz: P. zingiberensis, Gp: G. pentaphyllum.
By transcriptomic analysis and in vitro activity screening, other UGTs, namely OAGT, OAGT1, OAGT2, and OAGT3, particularly transferring glucuronic acid at the C-3 position of OA yielding calenduloside E, have also been characterized24. Three of them (OAGT1-3) were from P. zingiberensis and one (OAGT) was from P. japonicus var. major24 (Fig. 5D). Although UGTs glycosylating C28-carboxyl of OA have not been reported, UGT74M1 from Saponaria vaccaria could transfer UDP-Glc to C28-carboxyl of gypsogenic acid, a substrate with similar structure to OA148.
Whole genome sequencing of P. ginseng98,99 and P. notoginseng100,149,150 provided a genome-scale metabolic network and a holistic view of ginsenoside biosynthesis. The chromosome-level genome assembly and detailed transcriptional analysis of P. notoginseng combined with multi-omics analysis showed the biosynthesis and regulation of saponins at temporal and spatial levels, and would facilitate the identification of new functional genes including UGTs100. Transcriptomics of other Panax species24,151, 152, 153, 154, 155 have also been reported, which will prompt identification of new UGTs in those Panax species.
3.5. Acyltransferases
Ginsenoside are always malonylated at the hydroxyl groups of sugar chains to generate malonylginsenosides, represented by mRb1, mRb2, mRc, mRd, and mRe. This is the last process to diversify the structure of ginsenosides. None of the enzymes responsible for linking those malonyl groups to ginsenosides has been reported till now, although BAHD acyltransferases have been extensively reported in other plants156. For example, malonyltransferases, the BAHD family members using malonyl-CoA thioesters as the acyldonors, are versatile plant acyltransferases. Diverse malonyltransferases responsible for malonylating anthocyanin, flavonoid, isoflavonoid, and isoflavone glucosides have been functionally characterized in plants157, 158, 159, 160. Such work would benefit for the researches of acyltransferases in ginsenoside biosynthesis.
4. Regulators
As well known, the biosynthesis of plant secondary metabolites is controlled by sophisticated regulatory networks in which regulators such as transcription factors (TFs) and non-coding RNAs (ncRNAs) play important roles. Thus, exploring regulators in ginsenoside biosynthesis is very helpful to decipher the accumulation patterns of these compounds in ginseng species. It has been reported that some TFs and ncRNAs could be stimulated by biotic and abiotic signals and participated in the regulation of ginsenoside biosynthesis.
4.1. Transcription factors (TFs)
A total of 2150 transcription factors belonging to 57 different families were identified from P. notoginseng100, and 851 transcriptional regulators were predicted in P. ginseng99. TFs of WRKY, bHLH, MYB and AP2/ERF TF families play key roles in ginsenoside biosynthesis (Fig. 6). JA responsive genes involved in ginsenoside biosynthesis are regulated by different TFs161, 162, 163. MeJA and its analogues, the best effective elicitor on ginsenoside accumulation, were often used to stimulate ginsenoside biosynthesis in plant tissue cultures52. (+)-7-iso-Jasmonoyl-l-isoleucine (JA-Ile) was considered as the endogenous bioactive jasmonate164, and MeJA was cleaved by esterases and subsequently converted to JA-Ile by JA-amino acid synthetase (JAR1) before working as an elicitor165. Jasmonate ZIM-domain (JAZ) proteins are among the most important components of the jasmonate pathway, acting as both repressors of downstream TFs and co-receptors of the hormone, together with the COI1 receptor166. Crosstalk between JA and other hormones is beyond this topic and can be find elsewhere167,168.
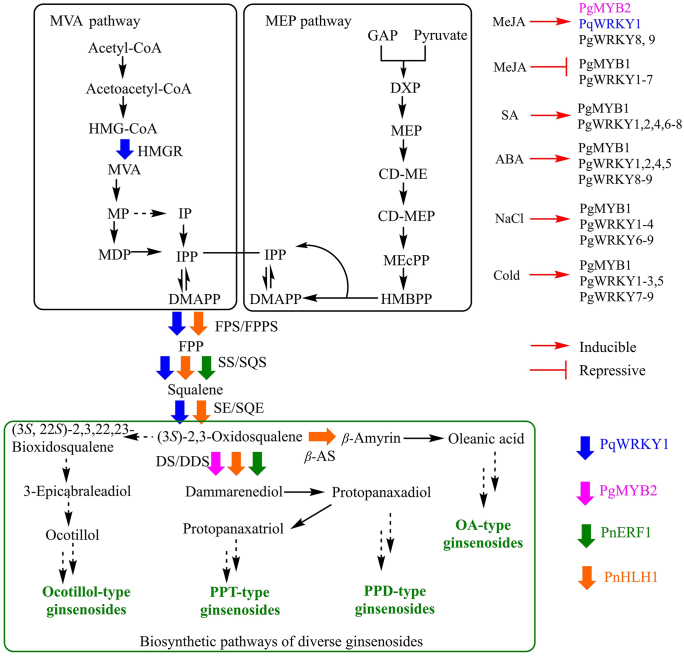
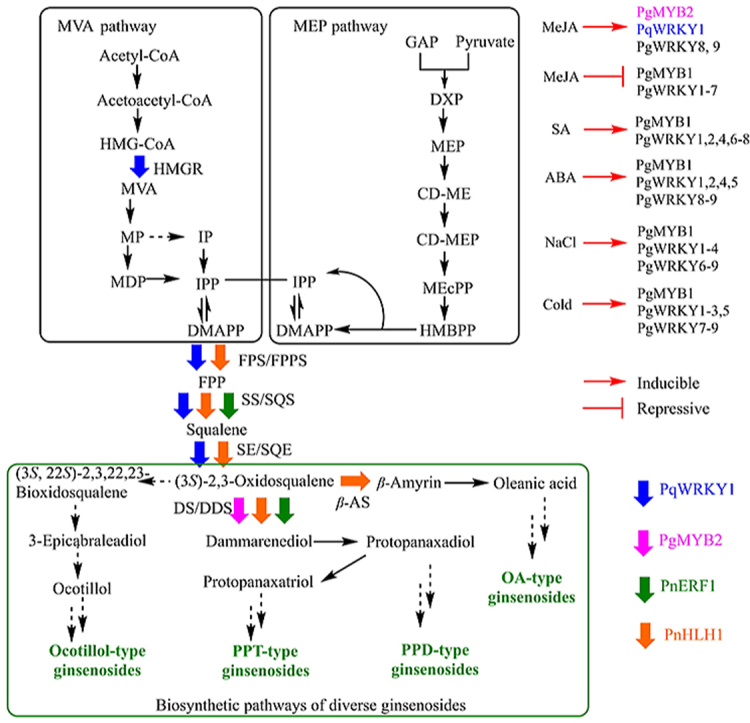
Figure 6.
Transcription factors identified involved in ginsenoside biosynthesis. HMGR, FPPS, SS, SE, β-AS and DDS are regulated by at least one TF from Panax species, TFs regulating other steps of ginsenoside biosynthesis is unresolved. The color of the hollow arrow corresponds to the color of the transcription factors, which regulate the enzyme encoding gene. The abbreviations are indicated as follows, β-AS: β-amyrin synthase, CD-ME: 4-diphosphocytidyl-2-C-methylerythritol, CD-MEP: 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate, DS/DDS: dammarenediol-II synthase, DMAPP: dimethylallyl diphosphate, DXP: 1-deoxyxylulose 5-phosphate, FPP: farnesyl diphosphate, FPS/FPPS: farnesyl diphosphate synthase, GAP: glyceraldehyde-3-phosphate, HMBPP: 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate, HMG-CoA (S)-3-hydroxy-3-methylglutaryl-coenzyme A, HMGR: HMG-CoA reductase, IP: isopentenyl phosphate, IPP: isopentenyl diphosphate, MDP: mevalonate-5-diphosphate, MEcPP: 2-C-methyl-d-erythritol-2,4-cyclodiphosphate, MEP: methylerythritol phosphate, MP: mevalonate-5-phosphate, MVA (3R)-3,5-dihydroxy-3-methylpentanoic acid, SS/SQS: squalene synthase, SE/SQE: squalene epoxidases.
Six PgbHLH genes were discovered to be potentially involved in the regulation of ginsenoside biosynthesis through tissue-specific expression and chemical constituents analysis162. In transgenic PnbHLH P. notoginseng cells, the expression levels of the four key genes involved in the biosynthesis of triterpenoid saponins namely, PnDS, PnSS, PnSE and PnFPS, were upregulated and the total saponin contents reached to about 2-fold in the transgenic PnbHLH1 cell lines compared with the wild cell lines169.
PqWRKY1, one of the WRKY family genes which could be induced by MeJA, was a positive regulator related to osmotic stress and ginsenoside biosynthesis in P. quinquefolius161. The expression level of HMGR, FPS2, SQS1, and SQE2 in transgenic PqWRKY1 Arabidopsis thaliana were upregulated to 1−5-fold of the control, and the salt and drought tolerance of the transgenic plant was increased correspondingly161. Nine PgWRKYs (PgWRKY1‒9) in P. ginseng have been identified recently, each of which includes one WRKYGQK sequence motif and one C2H2-type zinc-finger motif. Some of them could respond to cold, salt (NaCl) and various hormone external signals170,171. Notably, hypothermia stimulus experiments showed that the expression level of five ginsenoside biosynthetic genes viz., GPS, SS, CYP716A53v2, UGT74AE2 and UGT94Q2, and three PgWRKYs viz., PgWRKY1, PgWRKY3 and PgWRKY8 had a strong positive correlation with the production of total ginsenosides, suggesting these PgWRKYs might participate in the biosynthesis of ginsenosides through regulating the pathway genes171.
PgMYB1, a R2R3-type gene encoding a 238 amino acid protein, was expressed at higher level in roots, leaves, and lateral roots than in stems and seeds in P. ginseng. The transcription of PgMYB1 could be upregulated by ABA, SA, NaCl, and cold (chilling), and downregulated by MeJA172, while PgMYB2, another R2R3-type MYB gene, was significantly induced by MeJA and highly expressed in ginseng roots. PgMYB2 could bind to the promoter of DDS, and the transient expression of PgMYB2 in ginseng leaves promoted the expression of PgDDS163, indicating that PgMYB2 was possibly responsible for the biosynthesis of ginsenosides.
PnERF1, an AP2/ERF-type TF from P. notoginseng, was isolated by full-length cDNA cloning using Rapid Amplification of cDNA Ends (RACE) method173. In PnERF1-overexpressing P. notoginseng cell lines, the transcription levels of DS and SS were upregulated to 1.6- and 1.9-times of that in the control, and consequently six major monomer ginsenosides (Rg3, Rh1, Rd, Rg1, F1 and Re), especially Re and Rg1 were increased173, suggesting that PnERF1 was related to the biosynthesis of ginsenosides.
4.2. ncRNAs
As reported, a total of 3688 mRNA-like non-coding RNAs (mlncRNAs), a class of lncRNAs, were identified in P. ginseng. Approximately 40% of the identified mlncRNAs were processed into small RNAs, implying their regulatory roles via small RNA-mediated mechanisms174. 73 conserved microRNAs (miRNAs) belonging to 33 families, and 28 non-conserved ones belonging to 9 families were identified from P. ginseng. Five of them were dehydration-responsive and ten of them were heat responsive miRNAs, and there was a crosstalk among some of the stress-responsive miRNAs175. In P. notoginseng roots, miR156 and miR166 were considered as the largest miRNA families, while miR156i and miR156g were the highest abundant ones176. Another report showed that miR156 family and one of its Squamosa Promoter-Binding Protein-Like (SPL) target genes had inverse expression levels, which was tightly correlated with greater root biomass contents177.
5. Biotechnological approaches for ginsenoside production
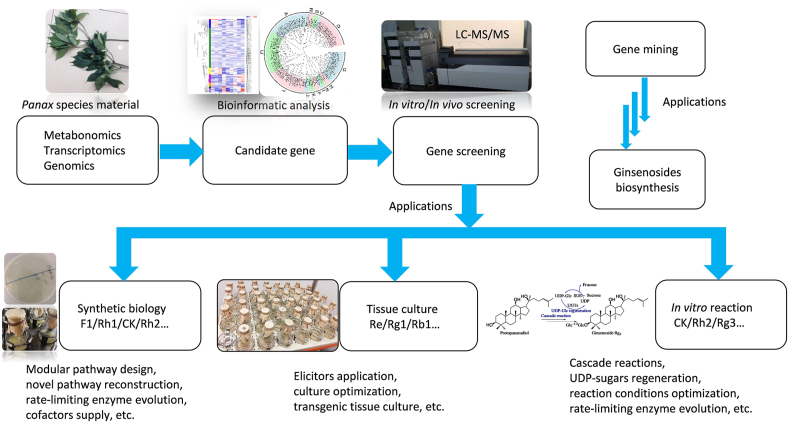
Recent advances in understanding the ginsenoside biosynthesis are now opening the way to access these biologically important compounds applying time-saving and low-cost biotechnologies. Plant cell and tissue culture, synthetic biology-based microbial cell factory, and in vitro cascade reactions have been developed in ginsenoside production in recent years (Fig. 7).
Figure 7.
Omics-based gene mining technology and their applications in ginsenoside biosynthesis.
5.1. Plant cell and tissue culture
Plant cell and tissue culture of ginseng is an effective approach for production of saponins51,55. Hairy roots (HR), adventitious roots (AR), and suspension cells (SC) were three commonly used materials. Generally, hairy roots were generated directly from explants infected by Agrobacterium rhizogenes178. Adventitious roots and suspension cells were induced from the explants via calli usually on Murashige and Skoog (MS) medium supplemented with plant growth regulators (PGRs), mostly 2,4-dichlorophenoxy acetic acid (2,4-D) or sometimes α-naphthalene acetic acid (NAA) and kinetin instead of 2,4-D for health and safety considerations50,51. Subculture of calli on liquid MS medium without any PGRs forms suspension cells179. Furthermore, adventitious roots were developed from calli cultured on MS medium supplemented with indole-3-butyric acid (IBA)180. Hairy root and adventitious root culture showed great advantages in both stable biomass growth and high ginsenoside production. However, hairy roots require A. rhizogenes induction and sustained antibiotics, which might have harmful effect to health. Currently, ginseng adventitious root culture has been industrialized with high ginsenoside yield and good safety51.
MS or modified MS media were mostly used in plant cell and tissue cultures of Panax species, while B5 and SH (Schenk and Hildebrandt) media were occasionally used53. Sucrose in the concentration of 3%–5% was preferred carbon source181. Macro-element concentrations and nitrogen source showed important effects on ginseng adventitious roots growth and ginsenoside accumulation in modified SH medium cultures182. Appropriate temperature and light intensity also have profound effects on biomass growth and ginsenoside accumulation. In a hairy root culture experiment, the optimum temperature for total ginsenosides production (10.5 mg/g DW, 133.4 mg/L) was at 25 °C and the optimum temperature for biomass growth was under 20 and 13 °C cycle of day (12 h) and night (8 h)183. Fluorescent light (FL) irradiation was good for ginsenoside production (30.2 ± 0.9 mg/L), while red and dark light were favorable for hairy root growth, so two stage bioreactor culture methodology was proposed183.
Application of elicitors are effective to improve ginsenoside production. Among these elicitors, MeJA and its analogues were most effective52. In a MeJA elicited experiment, all the MVA pathway genes except IDI were up-regulated. What's more, HMGR which encoded the enzyme that catalyzed the rate-limiting step in this pathway, was induced up to 178-fold in leaves and 6-fold in roots141. In hormone elicited experiments, two stage culture strategies were adopted to reduce the impact on biomass growth and get high content of ginsenoside. A two stage culture of P. notoginseng adventitious roots experiment showed that the highest total content of saponins (71.94 mg/g) was achieved after being treated with 5 mg/L JA, which was 2.09-fold higher than native roots and 8.45-fold higher than that in the control184. The ginsenoside content in adventitious roots of P. ginseng elicited with 150 μmol/L of MeJA showed up to 60 mg/g DW, which was about 20-fold enhancement compared to roots without MeJA induction185. MeJA or JA treatment can obviously increase ginsenoside content and change the ratio of PPD- and PPT-type ginsenosides179,185, probably by upregulating the expression level of CYP716A47 and CYP716A53v2 in a different degree125. Interestingly, both JA (5 mg/L) and methyl dihydrojasmonate (MDJ, 5 mg/L) treated P. notoginseng adventitious roots obviously increased both Rd and Rg group ginsenoside biosynthesis184. Another synthesized elicitor, 2-hydroxyethyl jasmonate (HEJ) could increase both protopanaxdiol 6-hydroxylase (P6H) and UDPG-ginsenoside Rd glucosyltransferase (UGRdGT) activities and simultaneously increased Rb1 and Rg group ginsenosides content in P. notoginseng cell cultures186. Abiotic elicitors such as, polyunsaturated fatty acids (PUFAs, JA precursor), ABA and SA187, 188, 189, and biotic elicitors such as, yeast extracts and microbes were also used to increase saponins content190, 191, 192. JA, reactive oxygen species (ROS), Ca+ and ethylene signaling are involved in ginsenoside biosynthesis as reviewed by Rahimi et al54.
Transgenic method is another effective way to improve ginsenoside production in tissue and cell culture. HMGR, SS, and FPS were three mostly used functional genes in transgenic ginseng cells and tissues. Overexpression of PnHMGR in P. notoginseng cells increased total ginsenoside content from about 20 to 40 mg/g, and co-overexpressions of PnHMGR and PnSS increased total ginsenoside titer to about 60 mg/g, which was about 2- and 3-times higher than that of the control, respectively92. In P. ginseng hairy roots, transgenic lines transformed with PgFPS showed the highest total ginsenoside content (36.42 mg/g), which was 2.4-fold higher compared with wild-type control94. Besides, transcription factors such as, PnbHLH1 and PnERF1 were also selected to improve ginsenoside biosynthesis. Transgenic cell lines of P. notoginseng with PnbHLH1 increased total ginsenoside content by 1.7–2.2-fold compared with that in control cell lines169. In another experiment, the content of total saponins in PnERF1 transgenic P. notoginseng increased about 2-fold from 40 mg/g in control cell lines to 80 mg/g in the top transgenic cell lines173. Transgenic ginseng plants with stable heredity and high saponin content productivity need to be further investigated. Main achievements of ginseng tissue and cell cultures in recent years are summarized in Table 292,94,169,173,178,184,186, 187, 188,191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215.
Table 2.
Recent achievements in the production of ginsenosides by plant cell and tissue cultures (from 2006 to 2020).
| No. | Plant | Cultured material | Basal medium | Elicitor/Transgenic gene | Maximum titers | Ref. |
|---|---|---|---|---|---|---|
| 1 | P. ginseng | AR | MS | MeJA, SA | 40.4 mg/g DW with MeJA, 30.7 mg/g DW with SA | 193 |
| 2 | P. ginseng | AR | MS | SA | About 1 mg/g DW | 194 |
| 3 | P. ginseng | AR | MS | Nitrogen-fixing bacteria | 105.6 mg/g DW | 192 |
| 4 | P. ginseng | AR | MS | Ethephon, MeJA | About 30 mg/g DW | 195 |
| 5 | P. ginseng | AR | MS | Gamma irradiation | 63.2 mg/L | 196 |
| 6 | P. ginseng | AR | MS | – | 15.9 mg/g DW | 197 |
| 7 | P. ginseng | AR | MS | MeJA | 50 mg/g DW | 198 |
| 8 | P. ginseng | AR | MS | – | 63 mg/L | 199 |
| 9 | P. ginseng | AR | MS | – | 15.1 mg/g DW | 200 |
| 10 | P. ginseng | HR | MS, SH | PgFPS | 36.4 mg/g DW | 94 |
| 11 | P. ginseng | HR | MS | Tween 80, Tween 20, MeJA | 60−80 mg/g DW | 201 |
| 12 | P. notoginseng | SC | MS | MeJA, 2-hydroxyethyl jasmonate | 28.9 mg/g DW (Re, Rg1, Rb1 and Rd) | 186 |
| 13 | P. ginseng | SC | MS | – | 13.6 mg/g DW | 200 |
| 14 | P. ginseng | SC | 67-V | N,Nʹ-dicyclohexylcarbodiimide (DCCD), sodium nitroprusside | 3.4 mg/g DW | 202 |
| 15 | P. ginseng | SC | 67-V | NH4VO3, NaVO3, VOSO4, NiSO4, CuSO4, MnSO4 | 5.6 mg/g DW | 203 |
| 16 | P. ginseng | SC | MS | Casein hydrolysate | 4.9% | 214 |
| 17 | P. japonicus | SC | MS | Casein hydrolysate | 3.2% | 214 |
| 18 | P. japonicus | SC | MS | – | 2%–3% | 204 |
| 19 | P. japonicus | SC | MS | Casein hydrolysate | 49.4 mg/g DW | 205 |
| 20 | P. notoginseng | AR | MS | JA, methyl dihydrojasmonate (MDJ) | 71.94 mg/g DW | 184 |
| 21 | P. notoginseng | Calli | MS | PnSS and PnHMGR | About 60 mg/g | 92 |
| 22 | P. notoginseng | SC | MS | 2-Hydroxyethyl jasmonate, MeJA | >40 mg/g DW | 206 |
| 23 | P. notoginseng | SC | MS | Phenobarbital | 56.4 mg/g calculated by Re and Rg1 | 207 |
| 24 | P. notoginseng | SC | MS | MeJA, HEJA | Rg1 (47.4 mg/L), Re (52.3 mg/L), Rb1 (190 mg/L), Rd (12.1 mg/L) | 208 |
| 25 | P. notoginseng | SC | MS | PnbHLH1 | About 90 mg/g | 169 |
| 26 | P. notoginseng | SC | MS | PnERF1 | About 80 mg/g | 173 |
| 27 | P. quinquefolius | AR | MS | 4 mg/L Alternaria panax extracts | 276 mg/L | 191 |
| 28 | P. quinquefolius | HR | B5 | trans-Anethole | 27.79 mg/g DW | 209 |
| 29 | P. quinquefolius | HR | MS, B5 | – | Crude ginsenoside 0.2 g/g DW | 178 |
| 30 | P. quinquefolius | HR | B5 | ABA | 14.4 mg/g DW | 187 |
| 31 | P. quinquefolius | SC | MS | SA | About 70.5 mg/L | 188 |
| 32 | P. quinquefolius | SC | MS | Inorganic salt or culture filtrates of microorganisms | Total ginsenoside content increased 2- (54.3 mg/L) to 3.2-fold compared with control | 210 |
| 33 | P. quinquefolius | SC | MS | Lactoalbumin hydrolysate, MeJA | 45.93 mg/L | 211 |
| 34 | P. quinquefolius | SC | MS | – | 30 mg/g DW | 212 |
| 35 | P. vietnamensis | AR | MS | JA, ABA, SA, YE, CH | M-R2 (2.83%), Rg1 (0.32%), Rb1 (0.85%) | 215 |
| 36 | P. vietnamensis | SC | MS | Yeast extract (YE), chitosan (CH) | – | 213 |
| 37 | P. sikkimensis | SC | MS | SA | 102.2 mg/L | 188 |
The abbreviations are indicated as follows, HR: hairy roots; AR: adventitious roots; SC: suspension cells.
5.2. Synthetic biology-based microbial cell factories
Synthetic biology-based microbe cell factories especially heterogeneous expression systems such as engineered yeast and Escherichia coli provide efficient tools for various natural products synthesis216, 217, 218. The identification of PgDDS and CYP716A47 was conducive to producing useful dammarane-type ginsenosides by genetic engineering approaches86,124, and with the identification of UGTPg1, the first glycosyltransferase in ginsenoside biosynthesis, ginsenoside compound K was produced with 1.4 mg/L in engineered yeast138. By co-expression of truncated HMGR (tHMGR), DDS and β-AS, OAS, PPDS, PPTS and A. thaliana cytochrome P450 reductase gene (AtCPR) in Saccharomyces cerevisiae, 17.2 mg/L PPD, 15.9 mg/L PPT and 21.4 mg/L oleanolic acid were produced, simultaneously219. Overexpression of tHMGR was an effect method to increased PPD production, and simultaneously overexpressing tHMGR, FPS, SS, SE and PPDS led to 262-fold increase of PPD production. Finally, 1.19 g/L PPD and 1.55 g/L DM were produced in engineered S. cerevisiae via two-phase extractive fermentation220. Increasing coupling efficiency between PPDS and ATR1 by protein fusion was another strategy to improve PPD production and reduce the ratio of DM to PPD221. PPD content reached up to 4.25 g/L in 5 L fed-batch fermentation by PPDS–ATR1 protein fusion and S. cerevisiae ROS tolerance enhancing222. Protein and metabolic engineering were two other good choices to improve ginsenoside titers. By introducing semi-rationally designed mutant glycosyltransferase gene into yeast and further metabolic engineering, including preventing Rh2 hydrolysis and increasing UDP-Glc precursor supply, ginsenoside Rh2 were produced at about 300 mg/L in a 5 L bioreactor by fed-batch fermentation223. In a recent study, firstly via modular engineering of the MVA pathway and optimization of CYPs expression levels, and then by increasing the copy numbers of UGTs and engineering its promoter to increase expression levels, at last through direct evolution of UGT bioparts, the highest titer of PPD and Rh2 was reached up to 11.02 and 2.25 g/L in 10 L fed-batch fermentation, respectively224. Moreover, biosynthesis of bioactive unnatural ginsenosides also made great success, the titers of 3β-O-Glc-DM and 20S-O-Glc-DM, two saponins with anti-colon cancer activities, were reached to 2.4 and 5.6 g/L through fed-batch fermentation, respectively225. Main achievements and strategies in ginsenoside biosynthesis are summarized in Table 3138,141,142,146,219, 220, 221, 222, 223, 224, 225, 226.
Table 3.
Recent achievements in ginsenoside biosynthesis in yeast cell factories.
| No. | Compd. | Titer (g/L) | Strategy | Ref. |
|---|---|---|---|---|
| 1 | CK | 1.4 × 10−3 | Coexpression or overexpression of DDS, CYP716A47, ATR2-1, UGTPg1, tHMGR and UPC2.1 | 138 |
| 2 | CK | 1.17 | Overexpression of FPPS, SS, SE and MVA pathway genes, codon optimization of Pn3-29, integration three copies of PgDDS, six copies of PgPPDS, two copies of VvCPR, insertion of Pn3-29 and UDP-glucose synthetic pathway genes into the chromosomal LPP1 site, inactivation of LPP1 genes, cloning Pn3-29 into the high-copy 2 micron plasmid | 146 |
| 3 | DM | 1.55 | Codon optimization of PPDS, coexpression or overexpression of tHMGR, FPPS, SS, SE, DDS, PPDS and AtCPR1 | 220 |
| PPD | 1.19 | |||
| 4 | DM | 7.10–8.09 | Modular engineering of MVA pathway, optimization of CYPs expression level, integrating multicopy of UGT, and engineering UGT promoter | 224 |
| PPD | 9.05–11.02 | |||
| Rh2 | 2.25 | |||
| 5 | 3β-O-Glc-DM | 2.4 | Chassis cell optimization, integrating multicopy of DDS and UGT, block competitive pathway, and overexpression rate-limiting enzymes and transcriptional activator | 225 |
| 20S-O-Glc-DM | 5.6 | |||
| 6 | 3β,12β-Di-O-Glc-PPD | 9.05 × 10−3 | Coexpression of DDS, PPDS, ATR1, UGT109A1 and overexpression of tHMGR | 226 |
| 7 | PPD | 17.2 × 10−3 | Coexpression of truncated HMGR (tHMGR), DDS and β-AS, OAS, PPDS, PPTS and AtCPR | 219 |
| PPT | 15.9 × 10−3 | |||
| OA | 21.4 × 10−3 | |||
| 8 | PPD | 1.44 | Construction of PPDS–ATR1 fusion protein, and overexpression of tHMGR, FPPS, SS and SE | 221 |
| 9 | PPD | 4.25 | Overexpression of tHMGR, FPPS, SS, SE, SSD1 and YBP1, and construction of PPDS–ATR1 fusion protein | 222 |
| 10 | Rg3 | 1.3 × 10−3 | Coexpression of DDS, PPDS, ATR2, UGT74AE2 and UGT 94Q2 and replace ERG7 promoter with methionine-repressible MET3 | 141 |
| 11 | Rh2 | 17.0 × 10−3 (1.5 μmol/g) | Coexpression of tHMGR, FPPS, SS, SE, DDS, PPDS, ATR2.1, UGTPg45 and UGTPg29 | 142 |
| Rg3 | 49.8 × 10−3 (3.5 μmol/g) | |||
| 12 | Rh2 | 0.3 | Directed evolution of UGT51, block Rh2 hydrolysis, and increasing UDP-Glc supply | 223 |
5.3. In vitro cascade reactions
In vitro enzymatic methods especially cascade reactions and in-site UDP-sugars regeneration systems provided a green chemistry approach for efficient glycosylation in natural products synthesis227,228. Ginsenoside Rh2 was synthesized in 0.20 g/L by coupling promiscuous glycosyltransferase and sucrose synthase in one-pot reactions, representing the first attempt of ginsenoside biosynthesis in vitro229. The buffers, co-solvents, different substrate and enzyme ratios were often considered in cascade reactions.
Biotechnology-based approaches are independent of climate, season, cropland, and don't need pesticides, herbicides, and chemical fertilizers, thus providing environmentally friendly alternative ways for producing secondary metabolites230. The three main methods described above have their own advantages and challenges. The products of plant cell and tissue culture are not only ginsenosides but also ginseng peptides and polysaccharides, which are similar to that of the natural herbs and have great advantages in producing functional foods, cosmetics and healthcare products. Besides, plant cell and tissue cultures do not need to completely elucidate the biosynthetic pathways of ginsenosides. The challenge of this approach is that safety concerns must be paid on the culture extracts when using as cosmetics and foods products. Under these circumstances, the toxicological evaluation of plant cell and tissue cultures is required51, and using food-conform culture medium and replacing synthetic phytohormones like 2,4-D with natural ones such as indole-3-acetic acid, zeatin is necessary230. Another challenge is that high quality facilities and instruments are required for large-scale cultures of plant cell and tissues. Synthetic biology-based approaches are dependent on the elucidation of the biosynthetic pathway of target ginsenosides and require elaborate design, construction and verification before application, but it is conductive to obtaining single compounds such as certain sapogenins and their monosaccharide products, which are the start materials for new drug development or additives of healthcare products142,224,225. Besides, it usually utilizes microbial cell factories which have sophisticated fermentation processes and facilities for large-scale production. Similarly, the merits of in vitro cascade reactions are to obtain single products for pharmaceutical industries, and are convenient to obtain a series of structural analogues to provide leading compounds for new drug research and development. This method brings forward higher requests to the stabilities and activities of enzymes and is dependent on natural resources or other strategies for starting materials.
6. Prospectives
Panax genus plants together with their secondary metabolites, mostly ginsenosides, are valuable resource for medicinal therapy and healthcare. Distinct multiple biological activities of structural diversity ginsenosides are resulted from both the specific triterpene skeletons and various sugar moieties. New functional enzymes, tissue specific regulators and various catalytic complexes (metabolons) are three possible factors that result in ginsenoside structure diversities.
β-AS which produce β-amyrin, the precursor of OA-type ginsenosides, is not uncommon in plant kingdoms. However, DDS that has high sequence similarity with β-AS is a new functional OSC in Panax genus, producing DM, the precursor of PPD- and PPT-type ginsenosides. Moreover, OCT-type ginsenosides, another kind of dammarane-type ginsenosides with a new skeleton that was different from PPD- and PPT-type ones, were discovered in P. vietnamensis and P. quinquefolius. These findings predict the possible existence of other specialized OSCs in Panax species. The diverse sugar chains of ginsenosides indicate different new functional UGTs may play key roles in ginsenoside glycosylation. UGTs responsible for the formation of ginsenoside Re, ginsenoside Ro, chikusetsusaponin IVa and majonoside R2 have not been fully characterized.
PPT-type ginsenosides, a kind of predominant bioactive compounds in P. notoginseng roots, are not present in leaves of P. notoginseng. The tissue distribution difference of PPT-type ginsenosides reminds us that PPTS may be regulated by tissue specific regulators in P. notoginseng. Another possibility is that two different metabolons may play roles in PPD and PPT biosynthesis, respectively.
Catalytic complexes which are not deeply researched are widespread in life cycles. The fact that all OCT-type ginsenosides are C-6 hydroxylated saponins tell us a catalytic complex may exist (maybe SE, OSC, CYPs and other partners form a catalytic complex) and play an important role in OCT-type ginsenoside biosynthesis. Identification of these metabolons will help us to manipulate ginsenoside biosynthesis.
Moreover, how do the enzymes of multigene family members coordinate to produce a particular saponin? How do the lipophilic aglycones and hydrophilic sugar donors transport between different organelles, tissues or organs and which organelle is the main accumulating and assembling location for UGTs catalyzed glycosylation? To comprehensively decipher the biosynthesis and regulatory mechanism of ginsenosides will benefit further biotechnological production of such valuable natural products.
Acknowledgments
We thank Dr. Xin Ju for his valuable comments on the manuscript. This work was supported by National Natural Science Foundation of China (No. 81673540, No. 81530096), Natural Science Foundation of Shanghai (No. 16ZR1434100, China), and Shanghai local Science and Technology Development Fund Program guided by the Central Government (YDZX20203100002948, China).
Author contributions
Zhengtao Wang and Shujuan Zhao gave the outline of the review and conducted the modifying work. Maoqi Hou retrieved the literatures and drafted the manuscript. Rufeng Wang participated in revising the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Shujuan Zhao, Email: zhaoshujuan@126.com, zhaoshujuan@shutcm.edu.cn.
Zhengtao Wang, Email: ztwang@shutcm.edu.cn.
References
- 1.Lee C., Wen J. Phylogeny of Panax using chloroplast trnC-trnD intergenic region and the utility of trnC-trnD in interspecific studies of plants. Mol Phylogenet Evol. 2004;31:894–903. doi: 10.1016/j.ympev.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S.K., Pandit M.K. A new species of panax L. (Araliaceae) from Sikkim himalaya, India. Syst Bot. 2009;34:434–438. [Google Scholar]
- 3.Duy N.V., Trieu L.N., Chinh N.D., Tran V.T. A new variety of panax (Araliaceae) from lam vien plateau, vietnam and its molecular evidence. Phytotaxa. 2016;277:47–58. [Google Scholar]
- 4.Pandey A.K., Ali M.A. Intraspecific variation in Panax assamicus Ban. populations based on internal transcribed spacer (ITS) sequences of nrDNA. Indian J Biotechnol. 2012;11:30–38. [Google Scholar]
- 5.Gurung B., Bhardwaj P.K., Rai A.K., Sahoo D. Major ginsenoside contents in rhizomes of Panax sokpayensis and Panax bipinnatifidus. Nat Prod Res. 2018;32:234–238. doi: 10.1080/14786419.2017.1343322. [DOI] [PubMed] [Google Scholar]
- 6.Tung N.H., Quang T.H., Ngan N.T.T., Minh C.V., Anh B.K., Long P.Q. Oleanolic triterpene saponins from the roots of Panax bipinnatifidus. Chem Pharm Bull. 2011;59:1417–1420. doi: 10.1248/cpb.59.1417. [DOI] [PubMed] [Google Scholar]
- 7.Chen W., Balan P., Popovich D.G. Comparison of ginsenoside components of various tissues of New Zealand forest-grown Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolium L.) Biomolecules. 2020;10:372. doi: 10.3390/biom10030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu S., Zou K., Fushimi H., Cai S., Komatsu K. Comparative study on triterpene saponins of ginseng drugs. Planta Med. 2004;70:666–677. doi: 10.1055/s-2004-827192. [DOI] [PubMed] [Google Scholar]
- 9.Wan J., Yang F., Li S., Wang Y., Cui X. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharmaceut Biomed Anal. 2006;41:1596–1601. doi: 10.1016/j.jpba.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Jia X.H., Wang C.Q., Liu J.H., Li X.W., Wang X., Shang M.Y. Comparative studies of saponins in 1‒3-year-old main roots, fibrous roots, and rhizomes of Panax notoginseng, and identification of different parts and growth-year samples. J Nat Med. 2013;67:339–349. doi: 10.1007/s11418-012-0691-6. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka O., Morita T., Kasai R., Kinouchi J., Sanada S., Ida Y. Study on saponins of rhizomes of Panax pseudo-ginseng subsp. himalaicus collected at Tzatogang and Pari-la, Bhutan-Himalaya. Chem Pharm Bull. 1985;33:2323–2330. [Google Scholar]
- 12.Morita T., Kasai R., Kohda H., Tanaka O., Zhou J., Yang T.R. Chemical and morphological study on Chinese Panax japonicus C. A. MEYER (Zhujie-Shen) Chem Pharm Bull. 1983;31:3205–3209. [Google Scholar]
- 13.Biswas T., Singh M., Mathur A.K., Mathur A. A dual purpose cell line of an Indian congener of ginseng-Panax sikkimensis with distinct ginsenoside and anthocyanin production profiles. Protoplasma. 2015;252:697–703. doi: 10.1007/s00709-014-0695-z. [DOI] [PubMed] [Google Scholar]
- 14.Lee T.M., Marderosian A.D. Two-dimensional TLC analysis of ginsenosides from root of dwarf ginseng (panax trifolius L.) Araliaceae. J Pharm Sci. 1981;70:89–91. doi: 10.1002/jps.2600700119. [DOI] [PubMed] [Google Scholar]
- 15.Le T.H.V., Lee G.J., Vu H.K.L., Kwon S.W., Nguyen N.K., Park J.H. Ginseng saponins in different parts of Panax vietnamensis. Chem Pharm Bull. 2015;63:950–954. doi: 10.1248/cpb.c15-00369. [DOI] [PubMed] [Google Scholar]
- 16.Venugopal N., Ahuja P. Relationship between age, size, fecundity and climatic factors in Panax wangianus an endangered medicinal plant in the sacred grove forest of North-East India. J Forestry Res. 2011;22:427–435. [Google Scholar]
- 17.Morita T., Tanaka O., Kohda H. Saponin composition of rhizomes of Panax japonicus collected in South Kyushu, Japan, and its significance in oriental traditional medicine. Chem Pharm Bull. 1985;33:3852–3858. doi: 10.1248/cpb.33.3852. [DOI] [PubMed] [Google Scholar]
- 18.Shu P.P., Li L.X., He Q.M., Pan J., Li X.L., Zhu M. Identification and quantification of oleanane triterpenoid saponins and potential analgesic and anti-inflammatory activities from the roots and rhizomes of Panax stipuleanatus. J Ginseng Res. 2021;45:305–315. doi: 10.1016/j.jgr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita T., Kong Y.C., But P.P.H., Ng K.H., Yip T.T., Kasai R. Saponins of plants of Panax species collected in Central Nepal and their chemotaxonomical significance. II. Chem Pharm Bull. 1986;34:4368–4372. doi: 10.1248/cpb.48.889. [DOI] [PubMed] [Google Scholar]
- 20.Baeg I.H., So S.H. The world ginseng market and the ginseng. J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B.R., Yuen S.C., Fan G.Y., Cong W.H., Leung S.W., Lee S.M.Y. Identification of certain Panax species to be potential substitutes for Panax notoginseng in hemostatic treatments. Pharmacol Res. 2018;134:1–15. doi: 10.1016/j.phrs.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S., Wang R., Zeng W., Zhu W., Zhang X., Wu C. Resource investigation of traditional medicinal plant Panax japonicus (T.Nees) C.A. Mey and its varieties in China. J Ethnopharmacol. 2015;166:79–85. doi: 10.1016/j.jep.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Yang J., Dong L.L., Wei G.F., Hu H.Y., Zhu G.W., Zhang J. Identification and quality analysis of Panax notoginseng and Panax vietnamensis var. fuscidicus through integrated DNA barcoding and HPLC. CHM. 2018;10:177–183. [Google Scholar]
- 24.Tang Q.Y., Chen G., Song W.L., Fan W., Wei K.H., He S.M. Transcriptome analysis of Panax zingiberensis identifies genes encoding oleanolic acid glucuronosyltransferase involved in the biosynthesis of oleanane-type ginsenosides. Planta. 2019;249:393–406. doi: 10.1007/s00425-018-2995-6. [DOI] [PubMed] [Google Scholar]
- 25.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Wang R.F., Zhou Y., Hu H.J., Yang Y.B., Yang L. Dammarane-type triterpene oligoglycosides from the leaves and stems of Panax notoginseng and their antiinflammatory activities. J Ginseng Res. 2019;43:377–384. doi: 10.1016/j.jgr.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Im D.S. Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of Panax ginseng. Biomolecules. 2020;10:444. doi: 10.3390/biom10030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y., Li J., Wang J., Li X., Li J., Chu S. Ginsenoside Rg1 prevent and treat inflammatory diseases: A review. Int Immunopharm. 2020;87:106805. doi: 10.1016/j.intimp.2020.106805. [DOI] [PubMed] [Google Scholar]
- 29.Wong A.S.T., Che C.M., Leung K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat Prod Rep. 2015;32:256–272. doi: 10.1039/c4np00080c. [DOI] [PubMed] [Google Scholar]
- 30.Sun M.Y., Ye Y., Xiao L., Duan X.Y., Zhang Y.M., Zhang H. Anticancer effects of ginsenoside Rg3. Int J Mol Med. 2017;39:507–518. doi: 10.3892/ijmm.2017.2857. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Chu S., Lin M., Gao Y., Liu Y., Yang S. Anticancer property of ginsenoside Rh2 from ginseng. Eur J Med Chem. 2020;203:112627. doi: 10.1016/j.ejmech.2020.112627. [DOI] [PubMed] [Google Scholar]
- 32.Zhou P., Xie W., He S., Sun Y., Meng X., Sun G. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. 2019;8:204. doi: 10.3390/cells8030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai L., Gao J., Wei F., Zhao J., Wang D., Wei J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front Pharmacol. 2018;9:423. doi: 10.3389/fphar.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabavi S.F., Sureda A., Habtemariam S., Nabavi S.M. Ginsenoside Rd and ischemic stroke; A short review of literatures. J Ginseng Res. 2015;39:299–303. doi: 10.1016/j.jgr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng L., Sun S., Xie L.H., Wicks S.M., Xie J.T. Ginsenoside Re: Pharmacological effects on cardiovascular system. Cardiovasc Ther. 2012;30:e183–e188. doi: 10.1111/j.1755-5922.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- 36.Gao Y., Chu S., Zhang Z., Chen N. Hepataprotective effects of ginsenoside Rg—a review. J Ethnopharmacol. 2017;206:178–183. doi: 10.1016/j.jep.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Shin J.H., Kwon H.W., Cho H.J., Rhee M.H., Park H.J. Vasodilator-stimulated phosphoprotein-phosphorylation by ginsenoside Ro inhibits fibrinogen binding to αIIb/β3 in thrombin-induced human platelets. J Ginseng Res. 2016;40:359–365. doi: 10.1016/j.jgr.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Liu T., Zhao J., He T., Chen H., Wang J. Phospholipase Cγ2 signalling contributes to the haemostatic effect of notoginsenoside Ft1. J Pharm Pharmacol. 2019;71:878–886. doi: 10.1111/jphp.13057. [DOI] [PubMed] [Google Scholar]
- 39.Tran Q.L., Adnyana I.K., Tezuka Y., Harimaya Y., Saiki I., Kurashige Y. Hepatoprotective effect of majonoside R2, the major saponin from Vietnamese ginseng (Panax vietnamensis) Planta Med. 2002;68:402–406. doi: 10.1055/s-2002-32069. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed T., Raza S.H., Maryam A., Setzer W.N., Braidy N., Nabavi S.F. Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res Bull. 2016;125:30–43. doi: 10.1016/j.brainresbull.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Li X.Y., Sun L.W., Zhao D.Q. Current status and problem-solving strategies for ginseng industry. Chin J Integr Med. 2019;25:883–886. doi: 10.1007/s11655-019-3046-2. [DOI] [PubMed] [Google Scholar]
- 42.Adil M., Jeong B.R. In vitro cultivation of Panax ginseng C.A. Meyer. Ind Crop Prod. 2018;122:239–251. [Google Scholar]
- 43.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T., Guo R., Zhou G., Zhou X., Kou Z., Sui F. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: A review. J Ethnopharmacol. 2016;188:234–258. doi: 10.1016/j.jep.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Xu C., Wang W., Wang B., Zhang T., Cui X., Pu Y. Analytical methods and biological activities of Panax notoginseng saponins: Recent trends. J Ethnopharmacol. 2019;236:443–465. doi: 10.1016/j.jep.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Choi H.K., Brinckmann J.A., Jiang X., Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): A review. J Chromatogr A. 2015;1426:1–15. doi: 10.1016/j.chroma.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Ju Z., Yang Y., Zhang Y., Yang L., Wang Z. Phytochemical analysis of Panax species: a review. J Ginseng Res. 2021;45:1–21. doi: 10.1016/j.jgr.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J., Xu Y., Yang J., Wang W., Zhang J., Zhang R. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J Ginseng Res. 2017;41:373–378. doi: 10.1016/j.jgr.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng M., Yi Y.X., Zhang T., Ding Y., Le J. Stereoisomers of saponins in Panax notoginseng (sanqi): A review. Front Pharmacol. 2018;9:188. doi: 10.3389/fphar.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paek K.Y., Murthy H.N., Hahn E.J., Zhong J.J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv Biochem Eng Biotechnol. 2009;113:151–176. doi: 10.1007/10_2008_31. [DOI] [PubMed] [Google Scholar]
- 51.Murthy H.N., Georgiev M.I., Kim Y.S., Jeong C.S., Kim S.J., Park S.Y. Ginsenosides: Prospective for sustainable biotechnological production. Appl Microbiol Biotechnol. 2014;98:6243–6254. doi: 10.1007/s00253-014-5801-9. [DOI] [PubMed] [Google Scholar]
- 52.Lu J., Li J., Wang S., Yao L., Liang W., Wang J. Advances in ginsenoside biosynthesis and metabolic regulation. Biotechnol Appl Biochem. 2018;65:514–522. doi: 10.1002/bab.1649. [DOI] [PubMed] [Google Scholar]
- 53.Gantait S., Mitra M., Chen J.T. Biotechnological interventions for ginsenosides production. Biomolecules. 2020;10:538. doi: 10.3390/biom10040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahimi S., Kim Y.J., Yang D.C. Production of ginseng saponins: Elicitation strategy and signal transductions. Appl Microbiol Biotechnol. 2015;99:6987–6996. doi: 10.1007/s00253-015-6806-8. [DOI] [PubMed] [Google Scholar]
- 55.Kim Y.J., Zhang D.B., Yang D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv. 2015;33:717–735. doi: 10.1016/j.biotechadv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Wen J., Zimmer E.A. Phylogeny and biogeography of Panax L. (the ginseng genus, Araliaceae) inferences from ITS sequences of nuclear ribosomal DNA. Mol Phylogenet Evol. 1996;6:167–177. doi: 10.1006/mpev.1996.0069. [DOI] [PubMed] [Google Scholar]
- 57.Choi H.K., Wen J. A phylogenetic analysis of Panax (Araliaceae) integrating cpDNA restriction site and nuclear rDNA ITS sequence data. Plant Systemat Evol. 2000;224:109–120. [Google Scholar]
- 58.Zhu S., Fushimi H., Cai S., Komatsu K. Phylogenetic relationship in the genus Panax: Inferred from chloroplast trnK gene and nuclear 18S rRNA gene sequences. Planta Med. 2003;69:647–653. doi: 10.1055/s-2003-41117. [DOI] [PubMed] [Google Scholar]
- 59.Zuo Y., Chen Z., Kondo K., Funamoto T., Wen J., Zhou S. DNA barcoding of Panax species. Planta Med. 2011;77:182–187. doi: 10.1055/s-0030-1250166. [DOI] [PubMed] [Google Scholar]
- 60.Zuo Y.J., Wen J., Ma J.S., Zhou S.L. Evolutionary radiation of the Panax bipinnatifidus species complex (Araliaceae) in the Sino-Himalayan region of eastern Asia as inferred from AFLP analysis. J Systemat Evol. 2015;53:210–220. [Google Scholar]
- 61.Zhou M., Yang G., Sun G., Guo Z., Gong X., Pan Y. Resolving complicated relationships of the Panax bipinnatifidus complex in southwestern China by RAD-seq data. Mol Phylogenet Evol. 2020;149:106851. doi: 10.1016/j.ympev.2020.106851. [DOI] [PubMed] [Google Scholar]
- 62.Zuo Y.J., Wen J., Zhou S.L. Intercontinental and intracontinental biogeography of the eastern Asian-Eastern North American disjunct Panax (the ginseng genus, Araliaceae), emphasizing its diversification processes in eastern Asia. Mol Phylogenet Evol. 2017;117:60–74. doi: 10.1016/j.ympev.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Shi F.X., Li M.R., Li Y.L., Jiang P., Zhang C., Pan Y.Z. The impacts of polyploidy, geographic and ecological isolations on the diversification of Panax (Araliaceae) BMC Plant Biol. 2015;15:297. doi: 10.1186/s12870-015-0669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y., Shi C., Liu C., Yu M., Qi Y., Li S. Saponins from Panax bipinnatifidus Seem.: New strategy of extraction, isolation, and evaluation of tyrosinase inhibitory activity based on mathematical calculations. J Chromatogr B. 2016;1039:79–87. doi: 10.1016/j.jchromb.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 65.Liu F., Ma N., He C., Hu Y., Li P., Chen M. Qualitative and quantitative analysis of the saponins in Panax notoginseng leaves using ultra-performance liquid chromatography coupled with time-of-flight tandem mass spectrometry and high performance liquid chromatography coupled with UV detector. J Ginseng Res. 2018;42:149–157. doi: 10.1016/j.jgr.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W., Qiao X., Li K., Fan J., Bo T., Guo D.A. Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm Sin B. 2016;6:568–575. doi: 10.1016/j.apsb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu S., Yang W.Z., Yao C.L., Shi X.J., Li J.Y., Lou Y. Malonylginsenosides with potential antidiabetic activities from the flower buds of Panax ginseng. J Nat Prod. 2017;80:899–908. doi: 10.1021/acs.jnatprod.6b00789. [DOI] [PubMed] [Google Scholar]
- 68.Shi X.J., Yang W.Z., Qiu S., Yao C.L., Shen Y., Pan H.Q. An in-source multiple collision-neutral loss filtering based nontargeted metabolomics approach for the comprehensive analysis of malonyl-ginsenosides from Panax ginseng, P. quinquefolius, and P. notoginseng. Anal Chim Acta. 2017;952:59–70. doi: 10.1016/j.aca.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 69.Li F., Lv C., Li Q., Wang J., Song D., Liu P. Chemical and bioactive comparison of flowers of Panax ginseng Meyer, Panax quinquefolius L., and Panax notoginseng Burk. J Ginseng Res. 2017;41:487–495. doi: 10.1016/j.jgr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y.S., Jin Y.P., Gao W., Xiao S.Y., Zhang Y.W., Zheng P.H. Complete 1H-NMR and 13C-NMR spectral assignment of five malonyl ginsenosides from the fresh flower buds of Panax ginseng. J Ginseng Res. 2016;40:245–250. doi: 10.1016/j.jgr.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin J.H., Park Y.J., Kim W., Kim D.O., Kim B.Y., Lee H. Change of ginsenoside profiles in processed ginseng by drying, steaming, and puffing. J Microbiol Biotechnol. 2019;29:222–229. doi: 10.4014/jmb.1809.09056. [DOI] [PubMed] [Google Scholar]
- 72.Yang L., Zou H., Gao Y., Luo J., Xie X., Meng W. Insights into gastrointestinal microbiota-generated ginsenoside metabolites and their bioactivities. Drug Metab Rev. 2020;52:125–138. doi: 10.1080/03602532.2020.1714645. [DOI] [PubMed] [Google Scholar]
- 73.Kim E.H., Kim W. An insight into ginsenoside metabolite compound K as a potential tool for skin disorder. Evid Based Complement Alternat Med. 2018;2018:8075870. doi: 10.1155/2018/8075870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma A., Lee H.J. Ginsenoside compound K: Insights into recent studies on pharmacokinetics and health-promoting activities. Biomolecules. 2020;10:1028. doi: 10.3390/biom10071028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elshafay A., Tinh N.X., Salman S., Shaheen Y.S., Othman E.B., Elhady M.T. Ginsenoside Rk1 bioactivity: A systematic review. Peer J. 2017;5:e3993. doi: 10.7717/peerj.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi P., Park J., Kim T., Park S.H., Kim Hk, Kang K.S. Improved anticancer effect of ginseng extract by microwave-assisted processing through the generation of ginsenosides Rg3, Rg5 and Rk1. J Funct Foods. 2015;14:613–622. [Google Scholar]
- 77.Jo S.K., Kim I.S., Yoon K.S., Yoon H.H., Yoo H.H. Preparation of ginsenosides Rg3, Rk1, and Rg5-selectively enriched ginsengs by a simple steaming process. Eur Food Res Technol. 2014;240:251–256. [Google Scholar]
- 78.He B., Chen P., Xie Y., Li S., Zhang X., Yang R. 20(R)-Ginsenoside Rg3 protects SH-SY5Y cells against apoptosis induced by oxygen and glucose deprivation/reperfusion. Bioorg Med Chem Lett. 2017;27:3867–3871. doi: 10.1016/j.bmcl.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 79.Lv Q., Rong N., Liu L.J., Xu X.L., Liu J.T., Jin F.X. Antitumoral activity of (20R)- and (20S)-ginsenoside Rh2 on transplanted hepatocellular carcinoma in mice. Planta Med. 2016;82:705–711. doi: 10.1055/s-0042-101764. [DOI] [PubMed] [Google Scholar]
- 80.Zhang E., Gao B., Yang L., Wu X., Wang Z. Notoginsenoside Ft1 promotes fibroblast proliferation via PI3K/Akt/mTOR signaling pathway and benefits wound healing in genetically diabetic mice. J Pharmacol Exp Ther. 2016;356:324–332. doi: 10.1124/jpet.115.229369. [DOI] [PubMed] [Google Scholar]
- 81.Shen K., Leung S.W.S., Ji L., Huang Y., Hou M., Xu A. Notoginsenoside Ft1 activates both glucocorticoid and estrogen receptors to induce endothelium-dependent, nitric oxide-mediated relaxations in rat mesenteric arteries. Biochem Pharmacol. 2014;88:66–74. doi: 10.1016/j.bcp.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 82.Wang R.F., Li J., Hu H.J., Li J., Yang Y.B., Yang L. Chemical transformation and target preparation of saponins in stems and leaves of Panax notoginseng. J Ginseng Res. 2018;42:270–276. doi: 10.1016/j.jgr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piao X.M., Huo Y., Kang J.P., Mathiyalagan R., Zhang H., Yang D.U. Diversity of ginsenoside profiles produced by various processing technologies. Molecules. 2020;25:4390. doi: 10.3390/molecules25194390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tam D.N.H., Truong D.H., Nguyen T.T.H., Quynh L.N., Tran L., Nguyen H.D. Ginsenoside Rh1: A systematic review of its pharmacological properties. Planta Med. 2018;84:139–152. doi: 10.1055/s-0043-124087. [DOI] [PubMed] [Google Scholar]
- 85.Han J.Y., In J.G., Kwon Y.S., Choi Y.E. Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry. 2010;71:36–46. doi: 10.1016/j.phytochem.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 86.Tansakul P., Shibuya M., Kushiro T., Ebizuka Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett. 2006;580:5143–5149. doi: 10.1016/j.febslet.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 87.Huang Z., Lin J., Cheng Z., Xu M., Guo M., Huang X. Production of oleanane-type sapogenin in transgenic rice via expression of β-amyrin synthase gene from Panax japonicus C. A. Mey. BMC Biotechnol. 2015;15:45. doi: 10.1186/s12896-015-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang D., Rong Q., Chen Y., Yuan Q., Shen Y., Guo J. Molecular cloning and functional analysis of squalene synthase (SS) in Panax notoginseng. Int J Biol Macromol. 2017;95:658–666. doi: 10.1016/j.ijbiomac.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 89.Zhao S., Wang L., Liu L., Liang Y., Sun Y., Wu J. Both the mevalonate and the non-mevalonate pathways are involved in ginsenoside biosynthesis. Plant Cell Rep. 2014;33:393–400. doi: 10.1007/s00299-013-1538-7. [DOI] [PubMed] [Google Scholar]
- 90.Dellas N., Thomas S.T., Manning G., Noel J.P. Discovery of a metabolic alternative to the classical mevalonate pathway. eLife. 2013;2 doi: 10.7554/eLife.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henry L.K., Gutensohn M., Thomas S.T., Noel J.P., Dudareva N. Orthologs of the archaeal isopentenyl phosphate kinase regulate terpenoid production in plants. Proc Natl Acad Sci U S A. 2015;112:10050–10055. doi: 10.1073/pnas.1504798112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng B., Zhang P., Ge F., Liu D.Q., Chen C.Y. Enhancement of triterpenoid saponins biosynthesis in Panax notoginseng cells by co-overexpressions of 3-hydroxy-3-methylglutaryl CoA reductase and squalene synthase genes. Biochem Eng J. 2017;122:38–46. [Google Scholar]
- 93.Kim Y.J., Lee O.R., Oh J.Y., Jang M.G., Yang D.C. Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme a reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol. 2014;165:373–387. doi: 10.1104/pp.113.222596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim Y.K., Kim Y.B., Uddin M.R., Lee S., Kim S.U., Park S.U. Enhanced triterpene accumulation in Panax ginseng hairy roots overexpressing mevalonate-5-pyrophosphate decarboxylase and farnesyl pyrophosphate synthase. ACS Synth Biol. 2014;3:773–779. doi: 10.1021/sb400194g. [DOI] [PubMed] [Google Scholar]
- 95.Niu Y., Luo H., Sun C., Yang T.J., Dong L., Huang L. Expression profiling of the triterpene saponin biosynthesis genes FPS, SS, SE, and DS in the medicinal plant Panax notoginseng. Gene. 2014;533:295–303. doi: 10.1016/j.gene.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 96.Xu S., Zhao C., Wen G., Zhang H., Zeng X., Liu Z. Longitudinal expression patterns of HMGR, FPS, SS, SE and DS and their correlations with saponin contents in green-purple transitional aerial stems of Panax notoginseng. Ind Crop Prod. 2018;119:132–143. [Google Scholar]
- 97.Kim T.D., Han J.Y., Huh G.H., Choi Y.E. Expression and functional characterization of three squalene synthase genes associated with saponin biosynthesis in Panax ginseng. Plant Cell Physiol. 2011;52:125–137. doi: 10.1093/pcp/pcq179. [DOI] [PubMed] [Google Scholar]
- 98.Xu J., Chu Y., Liao B., Xiao S., Yin Q., Bai R. Panax ginseng genome examination for ginsenoside biosynthesis. GigaScience. 2017;6:1–15. doi: 10.1093/gigascience/gix093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim N.H., Jayakodi M., Lee S.C., Choi B.S., Jang W., Lee J. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol J. 2018;16:1904–1917. doi: 10.1111/pbi.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang Z., Tu L., Yang W., Zhang Y., Hu T., Ma B. The chromosome-level reference genome assembly for Panax notoginseng and insights into ginsenoside biosynthesis. Plant Communications. 2021;2:100113. doi: 10.1016/j.xplc.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia P., Zheng Y., Liang Z. Structure and location studies on key enzymes in saponins biosynthesis of Panax notoginseng. Int J Mol Sci. 2019;20:6121. doi: 10.3390/ijms20246121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Augustin J.M., Kuzina V., Andersen S.B., Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry. 2011;72:435–457. doi: 10.1016/j.phytochem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 103.Xue Z., Duan L., Liu D., Guo J., Ge S., Dicks J. Divergent evolution of oxidosqualene cyclases in plants. New Phytol. 2012;193:1022–1038. doi: 10.1111/j.1469-8137.2011.03997.x. [DOI] [PubMed] [Google Scholar]
- 104.Thimmappa R., Geisler K., Louveau T., O'Maille P., Osbourn A. Triterpene biosynthesis in plants. Annu Rev Plant Biol. 2014;65:225–257. doi: 10.1146/annurev-arplant-050312-120229. [DOI] [PubMed] [Google Scholar]
- 105.Hu W., Liu N., Tian Y., Zhang L. Molecular cloning, expression, purification, and functional characterization of dammarenediol synthase from Panax ginseng. BioMed Res Int. 2013;2013:285740. doi: 10.1155/2013/285740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L., Zhao S.J., Cao H.J., Sun Y. The isolation and characterization of dammarenediol synthase gene from Panax quinquefolius and its heterologous co-expression with cytochrome P450 gene PqD12H in yeast. Funct Integr Genom. 2014;14:545–557. doi: 10.1007/s10142-014-0384-1. [DOI] [PubMed] [Google Scholar]
- 107.Kim O.T., Lee J.W., Bang K.H., Kim Y.C., Hyun D.Y., Cha S.W. Characterization of a dammarenediol synthase in Centella asiatica (L.) Urban. Plant Physiol Biochem. 2009;47:998–1002. doi: 10.1016/j.plaphy.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 108.Han J.Y., Kwon Y.S., Yang D.C., Jung Y.R., Choi Y.E. Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol. 2006;47:1653–1662. doi: 10.1093/pcp/pcl032. [DOI] [PubMed] [Google Scholar]
- 109.Shan H., Segura M.J.R., Wilson W.K., Lodeiro S., Matsuda S.P.T. Enzymatic cyclization of dioxidosqualene to heterocyclic triterpenes. J Am Chem Soc. 2005;127:18008–18009. doi: 10.1021/ja055822g. [DOI] [PubMed] [Google Scholar]
- 110.Andre C.M., Legay S., Deleruelle A., Nieuwenhuizen N., Punter M., Brendolise C. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids. New Phytol. 2016;211:1279–1294. doi: 10.1111/nph.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Z., Yeats T., Han H., Jetter R. Cloning and characterization of oxidosqualene cyclases from Kalanchoe daigremontiana: Enzymes catalyzing up to 10 rearrangement steps yielding friedelin and other triterpenoids. J Biol Chem. 2010;285:29703–29712. doi: 10.1074/jbc.M109.098871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abe I. Enzymatic synthesis of cyclic triterpenes. Nat Prod Rep. 2007;24:1311–1331. doi: 10.1039/b616857b. [DOI] [PubMed] [Google Scholar]
- 113.Jo H.J., Han J.Y., Hwang H.S., Choi Y.E. β-Amyrin synthase (EsBAS) and β-amyrin 28-oxidase (CYP716A244) in oleanane-type triterpene saponin biosynthesis in Eleutherococcus senticosus. Phytochemistry. 2017;135:53–63. doi: 10.1016/j.phytochem.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 114.Gao K., Wu S.R., Wang L., Xu Y.H., Wei J.H., Sui C. Cloning and analysis of β-amyrin synthase gene in Bupleurum chinense. Genes Genom. 2015;37:767–774. [Google Scholar]
- 115.Chen H., Liu Y., Zhang X., Zhan X., Liu C. Cloning and characterization of the gene encoding β-amyrin synthase in the glycyrrhizic acid biosynthetic pathway in Glycyrrhiza uralensis. Acta Pharm Sin B. 2013;3:416–424. [Google Scholar]
- 116.Yu F., Thamm A.M.K., Reed D., Villa-Ruano N., Quesada A.L., Gloria E.L. Functional characterization of amyrin synthase involved in ursolic acid biosynthesis in Catharanthus roseus leaf epidermis. Phytochemistry. 2013;91:122–127. doi: 10.1016/j.phytochem.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Um Y., Jin M.L., Lee D.Y., Kim C.K., Hong C.P., Lee Y. Functional characterization of the β-amyrin synthase gene involved in platycoside biosynthesis in Platycodon grandiflorum. Hortic Environ Biotechnol. 2017;58:613–619. [Google Scholar]
- 118.Kushiro T., Shibuya M., Masuda K., Ebizuka Y. Mutational studies on triterpene synthases: Engineering lupeo synthase into β-amyrin synthase. J Am Chem Soc. 2000;122:6816–6824. [Google Scholar]
- 119.Zhang X., Yu Y., Jiang S., Yu H., Xiang Y., Liu D. Oleanane-type saponins biosynthesis in Panax notoginseng via transformation of β-amyrin synthase gene from Panax japonicus. J Agric Food Chem. 2019;67:1982–1989. doi: 10.1021/acs.jafc.8b07183. [DOI] [PubMed] [Google Scholar]
- 120.Zhao C., Xu T., Liang Y., Zhao S., Ren L., Wang Q. Functional analysis of β-amyrin synthase gene in ginsenoside biosynthesis by RNA interference. Plant Cell Rep. 2015;34:1307–1315. doi: 10.1007/s00299-015-1788-7. [DOI] [PubMed] [Google Scholar]
- 121.Urlacher V.B., Girhard M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends Biotechnol. 2012;30:26–36. doi: 10.1016/j.tibtech.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 122.Bathe U., Tissier A. Cytochrome P450 enzymes: A driving force of plant diterpene diversity. Phytochemistry. 2019;161:149–162. doi: 10.1016/j.phytochem.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 123.Nelson D., Werck-Reichhart D. A P450-centric view of plant evolution. Plant J. 2011;66:194–211. doi: 10.1111/j.1365-313X.2011.04529.x. [DOI] [PubMed] [Google Scholar]
- 124.Han J.Y., Kim H.J., Kwon Y.S., Choi Y.E. The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2011;52:2062–2073. doi: 10.1093/pcp/pcr150. [DOI] [PubMed] [Google Scholar]
- 125.Han J.Y., Hwang H.S., Choi S.W., Kim H.J., Choi Y.E. Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol-II during ginsenoside biosynthesis in Panax Ginseng. Plant Cell Physiol. 2012;53:1535–1545. doi: 10.1093/pcp/pcs106. [DOI] [PubMed] [Google Scholar]
- 126.Han J.Y., Kim M.J., Ban Y.W., Hwang H.S., Choi Y.E. The involvement of β-amyrin 28-oxidase (CYP716A52v2) in oleanane-type ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2013;54:2034–2046. doi: 10.1093/pcp/pct141. [DOI] [PubMed] [Google Scholar]
- 127.Fukushima E.O., Seki H., Ohyama K., Ono E., Umemoto N., Mizutani M. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol. 2011;52:2050–2061. doi: 10.1093/pcp/pcr146. [DOI] [PubMed] [Google Scholar]
- 128.Carelli M., Biazzi E., Panara F., Tava A., Scaramelli L., Porceddu A. Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell. 2011;23:3070–3081. doi: 10.1105/tpc.111.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moses T., Pollier J., Faizal A., Apers S., Pieters L., Thevelein J.M. Unraveling the triterpenoid saponin biosynthesis of the African shrub Maesa lanceolata. Mol Plant. 2015;8:122–135. doi: 10.1016/j.molp.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 130.Tamura K., Seki H., Suzuki H., Kojoma M., Saito K., Muranaka T. CYP716A179 functions as a triterpene C-28 oxidase in tissue-cultured stolons of Glycyrrhiza uralensis. Plant Cell Rep. 2017;36:437–445. doi: 10.1007/s00299-016-2092-x. [DOI] [PubMed] [Google Scholar]
- 131.Huang L., Li J., Ye H., Li C., Wang H., Liu B. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus. Planta. 2012;236:1571–1581. doi: 10.1007/s00425-012-1712-0. [DOI] [PubMed] [Google Scholar]
- 132.Gantt R.W., Peltier-Pain P., Cournoyer W.J., Thorson J.S. Using simple donors to drive the equilibria of glycosyltransferase-catalyzed reactions. Nat Chem Biol. 2011;7:685–691. doi: 10.1038/nchembio.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bar-Peled M., O Neill M.A. Plant nucleotide sugar formation, interconversion, and salvage by sugar recycling. Annu Rev Plant Biol. 2011;62:127–155. doi: 10.1146/annurev-arplant-042110-103918. [DOI] [PubMed] [Google Scholar]
- 134.Tiwari P., Sangwan R.S., Sangwan N.S. Plant secondary metabolism linked glycosyltransferases: An update on expanding knowledge and scopes. Biotechnol Adv. 2016;34:714–739. doi: 10.1016/j.biotechadv.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Rahimi S., Kim J., Mijakovic I., Jung K.H., Choi G., Kim S.C. Triterpenoid-biosynthetic UDP-glycosyltransferases from plants. Biotechnol Adv. 2019;37:107394. doi: 10.1016/j.biotechadv.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 136.Seki H., Tamura K., Muranaka T. P450s and UGTs: Key players in the structural diversity of triterpenoid saponins. Plant Cell Physiol. 2015;56:1463–1471. doi: 10.1093/pcp/pcv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Osmani S.A., Bak S., Møller B.L. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry. 2009;70:325–347. doi: 10.1016/j.phytochem.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 138.Yan X., Fan Y., Wei W., Wang P., Liu Q., Wei Y. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014;24:770–773. doi: 10.1038/cr.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wei W., Wang P., Wei Y., Liu Q., Yang C., Zhao G. Characterization of Panax ginseng UDP-glycosyltransferases catalyzing protopanaxatriol and biosyntheses of bioactive ginsenosides F1 and Rh1 in metabolically engineered yeasts. Mol Plant. 2015;8:1412–1424. doi: 10.1016/j.molp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 140.Yang C., Li C., Wei W., Wei Y., Liu Q., Zhao G. The unprecedented diversity of UGT94-family UDP-glycosyltransferases in Panax plants and their contribution to ginsenoside biosynthesis. Sci Rep. 2020;10:15394. doi: 10.1038/s41598-020-72278-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung S.C., Kim W., Park S.C., Jeong J., Park M.K., Lim S. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014;55:2177–2188. doi: 10.1093/pcp/pcu147. [DOI] [PubMed] [Google Scholar]
- 142.Wang P., Wei Y., Fan Y., Liu Q., Wei W., Yang C. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab Eng. 2015;29:97–105. doi: 10.1016/j.ymben.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 143.Lu C., Zhao S.J., Wang X.S. Functional regulation of a UDP-glucosyltransferase gene (Pq3-O-UGT1) by RNA interference and overexpression in Panax quinquefolius. Plant Cell Tissue Organ Cult. 2017;129:445–456. [Google Scholar]
- 144.Lu C., Zhao S., Wei G., Zhao H., Qu Q. Functional regulation of ginsenoside biosynthesis by RNA interferences of a UDP-glycosyltransferase gene in Panax ginseng and Panax quinquefolius. Plant Physiol Biochem. 2017;111:67–76. doi: 10.1016/j.plaphy.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 145.Lu J., Yao L., Li J.X., Liu S.J., Hu Y.Y., Wang S.H. Characterization of UDP-glycosyltransferase involved in biosynthesis of ginsenosides Rg1 and Rb1 and identification of critical conserved amino acid residues for its function. J Agric Food Chem. 2018;66:9446–9455. doi: 10.1021/acs.jafc.8b02544. [DOI] [PubMed] [Google Scholar]
- 146.Wang D., Wang J., Shi Y., Li R., Fan F., Huang Y. Elucidation of the complete biosynthetic pathway of the main triterpene glycosylation products of Panax notoginseng using a synthetic biology platform. Metab Eng. 2020;61:131–140. doi: 10.1016/j.ymben.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 147.Yu L., Chen Y., Shi J., Wang R., Yang Y., Yang L. Biosynthesis of rare 20(R)-protopanaxadiol/protopanaxatriol type ginsenosides through Escherichia coli engineered with uridine diphosphate glycosyltransferase genes. J Ginseng Res. 2019;43:116–124. doi: 10.1016/j.jgr.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meesapyodsuk D., Balsevich J., Reed D.W., Covello P.S. Saponin biosynthesis in Saponaria vaccaria. cDNAs encoding β-amyrin synthase and a triterpene carboxylic acid glucosyltransferase. Plant Physiol. 2007;143:959–969. doi: 10.1104/pp.106.088484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen W., Kui L., Zhang G., Zhu S., Zhang J., Wang X. Whole-genome sequencing and analysis of the Chinese herbal plant Panax notoginseng. Mol Plant. 2017;10:899–902. doi: 10.1016/j.molp.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 150.Zhang D., Li W., Xia E.H., Zhang Q.J., Liu Y., Zhang Y. The medicinal herb Panax notoginseng genome provides insights into ginsenoside biosynthesis and genome evolution. Mol Plant. 2017;10:903–907. doi: 10.1016/j.molp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 151.Rai A., Yamazaki M., Takahashi H., Nakamura M., Kojoma M., Suzuki H. RNA-seq transcriptome analysis of Panax japonicus, and its comparison with other Panax species to identify potential genes involved in the saponins biosynthesis. Front Plant Sci. 2016;7:481. doi: 10.3389/fpls.2016.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang J., Li J., Li J., Liu S., Wu X., Li J. Transcriptome profiling shows gene regulation patterns in ginsenoside pathway in response to methyl jasmonate in Panax quinquefolium adventitious root. Sci Rep. 2016;6:37263. doi: 10.1038/srep37263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang G.H., Ma C.H., Zhang J.J., Chen J.W., Tang Q.Y., He M.H. Transcriptome analysis of Panax vietnamensis var. fuscidicus discovers putative ocotillol-type ginsenosides biosynthesis genes and genetic markers. BMC Genom. 2015;16:159. doi: 10.1186/s12864-015-1332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gurung B., Bhardwaj P.K., Talukdar N.C. Subtractive transcriptome analysis of leaf and rhizome reveals differentially expressed transcripts in Panax sokpayensis. Funct Integr Genom. 2016;16:619–639. doi: 10.1007/s10142-016-0517-9. [DOI] [PubMed] [Google Scholar]
- 155.Wei G., Yang F., Wei F., Zhang L., Gao Y., Qian J. Metabolomes and transcriptomes revealed the saponin distribution in root tissues of Panax quinquefolius and Panax notoginseng. J Ginseng Res. 2020;44:757–769. doi: 10.1016/j.jgr.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bontpart T., Cheynier V., Ageorges A., Terrier N. BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds. New Phytol. 2015;208:695–707. doi: 10.1111/nph.13498. [DOI] [PubMed] [Google Scholar]
- 157.Paulsmeyer M.N., Brown P.J., Juvik J.A. Discovery of anthocyanin acyltransferase1 (AAT1) in maize using genotyping-by-sequencing (GBS) G3. 2018;8:3669–3678. doi: 10.1534/g3.118.200630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim D.H., Kim S.K., Kim J.H., Kim B.G., Ahn J.H. Molecular characterization of flavonoid malonyltransferase from Oryza sativa. Plant Physiol Biochem. 2009;47:991–997. doi: 10.1016/j.plaphy.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 159.Dhaubhadel S., Farhangkhoee M., Chapman R. Identification and characterization of isoflavonoid specific glycosyltransferase and malonyltransferase from soybean seeds. J Exp Bot. 2008;59:981–994. doi: 10.1093/jxb/ern046. [DOI] [PubMed] [Google Scholar]
- 160.Ahmad M.Z., Li P., Wang J., Rehman N.U., Zhao J. Isoflavone malonyltransferases GmIMaT1 and GmIMaT3 differently modify isoflavone glucosides in soybean (Glycine max) under various stresses. Front Plant Sci. 2017;8:735. doi: 10.3389/fpls.2017.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun Y., Niu Y., Xu J., Li Y., Luo H., Zhu Y. Discovery of WRKY transcription factors through transcriptome analysis and characterization of a novel methyl jasmonate-inducible PqWRKY1 gene from Panax quinquefolius. Plant Cell Tissue Organ Cult. 2013;114:269–277. [Google Scholar]
- 162.Chu Y., Xiao S., Su H., Liao B., Zhang J., Xu J. Genome-wide characterization and analysis of bHLH transcription factors in Panax ginseng. Acta Pharm Sin B. 2018;8:666–677. doi: 10.1016/j.apsb.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu T., Luo T., Guo X., Zou X., Zhou D., Afrin S. PgMYB2, a MeJA-responsive transcription factor, positively regulates the dammarenediol synthase gene expression in Panax ginseng. Int J Mol Sci. 2019;20:2219. doi: 10.3390/ijms20092219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fonseca S., Chini A., Hamberg M., Adie B., Porzel A., Kramell R. (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol. 2009;5:344–350. doi: 10.1038/nchembio.161. [DOI] [PubMed] [Google Scholar]
- 165.Staswick P.E., Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Monte I., Franco-Zorrilla J.M., García-Casado G., Zamarreño A.M., García-Mina J.M., Nishihama R. A single JAZ repressor controls the jasmonate pathway in Marchantia polymorpha. Mol Plant. 2019;12:185–198. doi: 10.1016/j.molp.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 167.Robert-Seilaniantz A., Grant M., Jones J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–343. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- 168.Ku Y.S., Sintaha M., Cheung M.Y., Lam H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci. 2018;19:3206. doi: 10.3390/ijms19103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang X., Ge F., Deng B., Shah T., Huang Z., Liu D. Molecular cloning and characterization of PnbHLH1 transcription factor in Panax notoginseng. Molecules. 2017;22:1268. doi: 10.3390/molecules22081268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiu H., Nuruzzaman M., Guo X., Cao H., Huang J., Chen X. Molecular cloning and expression analysis of eight PgWRKY genes in Panax ginseng responsive to salt and hormones. Int J Mol Sci. 2016;17:319. doi: 10.3390/ijms17030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang S., Liang W., Yao L., Wang J., Gao W. Effect of temperature on morphology, ginsenosides biosynthesis, functional genes, and transcriptional factors expression in Panax ginseng adventitious roots. J Food Biochem. 2019;43 doi: 10.1111/jfbc.12794. [DOI] [PubMed] [Google Scholar]
- 172.Afrin S., Zhu J., Cao H., Huang J., Xiu H., Luo T. Molecular cloning and expression profile of an abiotic stress and hormone responsive MYB transcription factor gene from Panax ginseng. Acta Biochim Biophys Sin. 2015;47:267–277. doi: 10.1093/abbs/gmv012. [DOI] [PubMed] [Google Scholar]
- 173.Deng B., Huang Z., Ge F., Liu D., Lu R., Chen C. An AP2/ERF family transcription factor PnERF1 raised the biosynthesis of saponins in Panax notoginseng. J Plant Growth Regul. 2017;36:691–701. [Google Scholar]
- 174.Wang M., Wu B., Chen C., Lu S. Identification of mRNA-like non-coding RNAs and validation of a mighty one named MAR in Panax ginseng. J Integr Plant Biol. 2015;57:256–270. doi: 10.1111/jipb.12239. [DOI] [PubMed] [Google Scholar]
- 175.Wu B., Wang M., Ma Y., Yuan L., Lu S. High-throughput sequencing and characterization of the small RNA transcriptome reveal features of novel and conserved microRNAs in Panax ginseng. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wei R., Qiu D., Wilson I.W., Zhao H., Lu S., Miao J. Identification of novel and conserved microRNAs in Panax notoginseng roots by high-throughput sequencing. BMC Genom. 2015;16:835. doi: 10.1186/s12864-015-2010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zheng Y., Chen K., Xu Z., Liao P., Zhang X., Liu L. Small RNA profiles from Panax notoginseng roots differing in sizes reveal correlation between miR156 abundances and root biomass levels. Sci Rep. 2017;7:9418. doi: 10.1038/s41598-017-09670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mathur A., Gangwar A., Mathur A.K., Verma P., Uniyal G.C., Lal R.K. Growth kinetics and ginsenosides production in transformed hairy roots of American ginseng-Panax quinquefolium L. Biotechnol Lett. 2010;32:457–461. doi: 10.1007/s10529-009-0158-3. [DOI] [PubMed] [Google Scholar]
- 179.Thanh N.T., Murthy H.N., Yu K.W., Hahn E.J., Paek K.Y. Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-L balloon type bubble bioreactors. Appl Microbiol Biotechnol. 2005;67:197–201. doi: 10.1007/s00253-004-1759-3. [DOI] [PubMed] [Google Scholar]
- 180.Gao X., Zhu C., Jia W., Gao W., Qiu M., Zhang Y. Induction and characterization of adventitious roots directly from the explants of Panax notoginseng. Biotechnol Lett. 2005;27:1771–1775. doi: 10.1007/s10529-005-3553-4. [DOI] [PubMed] [Google Scholar]
- 181.Wu J., Zhong J.J. Production of ginseng and its bioactive components in plant cell culture: Current technological and applied aspects. J Biotechnol. 1999;68:89–99. doi: 10.1016/s0168-1656(98)00195-3. [DOI] [PubMed] [Google Scholar]
- 182.Yu K.W., Gao W.Y., Hahn E.J., Paek K.Y. Effects of macro elements and nitrogen source on adventitious root growth and ginsenoside production in ginseng. J Plant Biol. 2001;44:179–184. [Google Scholar]
- 183.Yu K.W., Murthy H.N., Hahn E.J., Paek K.Y. Ginsenoside production by hairy root cultures of Panax ginseng: Influence of temperature and light quality. Biochem Eng J. 2005;23:53–56. [Google Scholar]
- 184.Li J., Wang J., Wu X., Liu D., Li J., Li J. Jasmonic acid and methyl dihydrojasmonate enhance saponin biosynthesis as well as expression of functional genes in adventitious roots of Panax notoginseng F.H. Chen. Biotechnol Appl Biochem. 2017;64:225–238. doi: 10.1002/bab.1477. [DOI] [PubMed] [Google Scholar]
- 185.Kim Y.S., Hahn E.J., Murthy H.N., Paek K.Y. Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett. 2004;26:1619–1622. doi: 10.1007/s10529-004-3183-2. [DOI] [PubMed] [Google Scholar]
- 186.Hu F.X., Zhong J.J. Role of jasmonic acid in alteration of ginsenoside heterogeneity in elicited cell cultures of Panax notoginseng. J Biosci Bioeng. 2007;104:513–516. doi: 10.1263/jbb.104.513. [DOI] [PubMed] [Google Scholar]
- 187.Kochan E., Balcerczak E., Szymczyk P., Sienkiewicz M., Zielińska-Bliźniewska H., Szymańska G. Abscisic acid regulates the 3-hydroxy-3-methylglutaryl CoA reductase gene promoter and ginsenoside production in Panax quinquefolium hairy root cultures. Int J Mol Sci. 2019;20:1310. doi: 10.3390/ijms20061310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Biswas T., Mathur A., Gupta V., Singh M., Mathur A.K. Salicylic acid and ultrasonic stress modulated gene expression and ginsenoside production in differentially affected Panax quinquefolius (L.) and Panax sikkimensis (Ban.) cell suspensions. Plant Cell Tissue Organ Cult. 2018;136:575–588. [Google Scholar]
- 189.Dewir Y.H., Chakrabarty D., Wu C.H., Hahn E.J., Jeon W.K., Paek K.Y. Influences of polyunsaturated fatty acids (PUFAs) on growth and secondary metabolite accumulation in Panax ginseng C.A. Meyer adventitious roots cultured in air-lift bioreactors. S Afr J Bot. 2010;76:354–358. [Google Scholar]
- 190.Kochan E., Szymczyk P., Kuźma Ł., Lipert A., Szymańska G. Yeast extract stimulates ginsenoside production in hairy root cultures of American ginseng cultivated in shake flasks and nutrient sprinkle bioreactors. Molecules. 2017;22:880. doi: 10.3390/molecules22060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu Y., Zhang W.B., Li X.Y., Piao X.C., Jiang J., Lian M.L. Pathogenic fungal elicitors enhance ginsenoside biosynthesis of adventitious roots in Panax quinquefolius during bioreactor culture. Ind Crop Prod. 2016;94:729–735. [Google Scholar]
- 192.Le K.C., Im W.T., Paek K.Y., Park S.Y. Biotic elicitation of ginsenoside metabolism of mutant adventitious root culture in Panax ginseng. Appl Microbiol Biotechnol. 2018;102:1687–1697. doi: 10.1007/s00253-018-8751-9. [DOI] [PubMed] [Google Scholar]
- 193.Ali M.B., Yu K.W., Hahn E.J., Paek K.Y. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006;25:613–620. doi: 10.1007/s00299-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 194.Tewari R.K., Paek K.Y. Salicylic acid-induced nitric oxide and ROS generation stimulate ginsenoside accumulation in Panax ginseng roots. J Plant Growth Regul. 2011;30:396–404. [Google Scholar]
- 195.Bae K.H., Choi Y.E., Shin C.G., Kim Y.Y., Kim Y.S. Enhanced ginsenoside productivity by combination of ethephon and methyl jasmoante in ginseng (Panax ginseng C.A. Meyer) adventitious root cultures. Biotechnol Lett. 2006;28:1163–1166. doi: 10.1007/s10529-006-9071-1. [DOI] [PubMed] [Google Scholar]
- 196.Le K.C., Ho T.T., Paek K.Y., Park S.Y. Low dose gamma radiation increases the biomass and ginsenoside content of callus and adventitious root cultures of wild ginseng (Panax ginseng Mayer) Ind Crop Prod. 2019;130:16–24. [Google Scholar]
- 197.Huang T., Gao W.Y., Wang J., Cao Y., Zhao Y.X., Huang L.Q. Selection and optimization of a high-producing tissue culture of Panax ginseng C. A. Meyer. Acta Physiol Plant. 2010;32:765–772. [Google Scholar]
- 198.Kim Y.S., Yeung E.C., Hahn E.J., Paek K.Y. Combined effects of phytohormone, indole-3-butyric acid, and methyl jasmonate on root growth and ginsenoside production in adventitious root cultures of Panax ginseng C.A. Meyer. Biotechnol Lett. 2007;29:1789–1792. doi: 10.1007/s10529-007-9442-2. [DOI] [PubMed] [Google Scholar]
- 199.Sivakumar G., Yu K.W., Paek K.Y. Enhanced production of bioactive ginsenosides from adventitious roots of Panax ginseng in bioreactor culture. J Hortic Sci Biotechnol. 2006;81:549–552. [Google Scholar]
- 200.Le K.C., Jeong C.S., Lee H., Paek K.Y., Park S.Y. Ginsenoside accumulation profiles in long- and short-term cell suspension and adventitious root cultures in Panax ginseng. Hortic Environ Biotechnol. 2019;60:125–134. [Google Scholar]
- 201.Liang Y., Wu J., Li Y., Li J., Ouyang Y., He Z. Enhancement of ginsenoside biosynthesis and secretion by tween 80 in Panax ginseng hairy roots. Biotechnol Appl Biochem. 2015;62:193–199. doi: 10.1002/bab.1256. [DOI] [PubMed] [Google Scholar]
- 202.Huang C., Qian Z.G., Zhong J.J. Enhancement of ginsenoside biosynthesis in cell cultures of Panax ginseng by N,N′-dicyclohexylcarbodiimide elicitation. J Biotechnol. 2013;165:30–36. doi: 10.1016/j.jbiotec.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 203.Huang C., Zhong J.J. Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process Biochem. 2013;48:1227–1234. doi: 10.1016/j.jbiotec.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 204.Demidova E.V., Reshetnyak O.V., Oreshnikov A.V., Nosov A.M. Growth and biosynthetic characteristics of ginseng (Panax japonicus var. repens) deep-tank cell culture in bioreactors. Russ J Plant Physl. 2006;53:134–140. [Google Scholar]
- 205.Kochkin D.V., Kachala V.V., Shashkov A.S., Chizhov A.O., Chirva V.Y., Nosov A.M. Malonyl-ginsenoside content of a cell-suspension culture of Panax japonicus var. repens. Phytochemistry. 2013;93:18–26. doi: 10.1016/j.phytochem.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 206.Hu F.X., Zhong J.J. Jasmonic acid mediates gene transcription of ginsenoside biosynthesis in cell cultures of Panax notoginseng treated with chemically synthesized 2-hydroxyethyl jasmonate. Process Biochem. 2008;43:113–118. [Google Scholar]
- 207.Yue C.J., Zhong J.J. Manipulation of ginsenoside heterogeneity of Panax notoginseng cells in flask and bioreactor cultivations with addition of phenobarbital. Bioproc Biosyst Eng. 2008;31:95–100. doi: 10.1007/s00449-007-0150-z. [DOI] [PubMed] [Google Scholar]
- 208.Wang W., Zhao Z.J., Xu Y.F., Qian X.H., Zhong J.J. Efficient induction of ginsenoside biosynthesis and alteration of ginsenoside heterogeneity in cell cultures of Panax notoginseng by using chemically synthesized 2-hydroxyethyl jasmonate. Appl Microbiol Biotechnol. 2006;70:298–307. doi: 10.1007/s00253-005-0089-4. [DOI] [PubMed] [Google Scholar]
- 209.Kochan E., Szymczyk P., Kuźma Ł., Szymańska G., Wajs-Bonikowska A., Bonikowski R. The increase of triterpene saponin production induced by trans-anethole in hairy root cultures of Panax quinquefolium. Molecules. 2018;23:2674. doi: 10.3390/molecules23102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Biswas T., Kalra A., Mathur A.K., Lal R.K., Singh M., Mathur A. Elicitors' influenced differential ginsenoside production and exudation into medium with concurrent Rg3/Rh2 panaxadiol induction in Panax quinquefolius cell suspensions. Appl Microbiol Biotechnol. 2016;100:4909–4922. doi: 10.1007/s00253-015-7264-z. [DOI] [PubMed] [Google Scholar]
- 211.Wang J., Gao W.Y., Zhang J., Huang T., Wen T.T., Huang L.Q. Combination effect of lactoalbumin hydrolysate and methyl jasmonate on ginsenoside and polysaccharide production in Panax quinquefolium L. cells cultures. Acta Physiol Plant. 2010;33:861–866. [Google Scholar]
- 212.Kochan E., Chmiel A. Dynamics of ginsenoside biosynthesis in suspension culture of Panax quinquefolium. Acta Physiol Plant. 2011;33:911–915. [Google Scholar]
- 213.Trong T.T., Truong D.H., Nguyen H.C., Tran D.T., Nguyen Thi H.T., Dang G.D. Biomass accumulation of Panax vietnamensis in cell suspension cultures varies with addition of plant growth regulators and organic additives. Asian Pac J Trop Med. 2017;10:907–915. doi: 10.1016/j.apjtm.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 214.Smirnova Y.N., Reshetnyak O.V., Smolenskaya I.N., Voevudskaya S.Y., Nosov A.M. Effect of growth regulators on ginsenoside production in the cell culture of two ginseng species. Russ J Plant Physl. 2010;57:430–437. [Google Scholar]
- 215.Linh N.T.N., Cuong L.K., Tam H.T., Tung H.T., Luan V.Q., Hien V.T. Improvement of bioactive saponin accumulation in adventitious root cultures of Panax vietnamensis via culture periods and elicitation. Plant Cell Tissue Organ Cult. 2019;137:101–113. [Google Scholar]
- 216.Paddon C.J., Westfall P.J., Pitera D.J., Benjamin K., Fisher K., McPhee D. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 217.Luo X., Reiter M.A., d'Espaux L., Wong J., Denby C.M., Lechner A. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature. 2019;567:123–126. doi: 10.1038/s41586-019-0978-9. [DOI] [PubMed] [Google Scholar]
- 218.Li Z., Wang X., Zhang H. Balancing the non-linear rosmarinic acid biosynthetic pathway by modular co-culture engineering. Metab Eng. 2019;54:1–11. doi: 10.1016/j.ymben.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 219.Dai Z., Wang B., Liu Y., Shi M., Wang D., Zhang X. Producing aglycons of ginsenosides in bakers' yeast. Sci Rep. 2014;4:3698. doi: 10.1038/srep03698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dai Z., Liu Y., Zhang X., Shi M., Wang B., Wang D. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng. 2013;20:146–156. doi: 10.1016/j.ymben.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 221.Zhao F., Bai P., Liu T., Li D., Zhang X., Lu W. Optimization of a cytochrome P450 oxidation system for enhancing protopanaxadiol production in Saccharomyces cerevisiae. Biotechnol Bioeng. 2016;113:1787–1795. doi: 10.1002/bit.25934. [DOI] [PubMed] [Google Scholar]
- 222.Zhao F., Dua Y., Bai P., Liu J., Lu W., Yuan Y. Enhancing Saccharomyces cerevisiae reactive oxygen species and ethanol stress tolerance for high-level production of protopanoxadiol. Bioresour Technol. 2017;227:308–316. doi: 10.1016/j.biortech.2016.12.061. [DOI] [PubMed] [Google Scholar]
- 223.Zhuang Y., Yang G.Y., Chen X., Liu Q., Zhang X., Deng Z. Biosynthesis of plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme. Metab Eng. 2017;42:25–32. doi: 10.1016/j.ymben.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 224.Wang P., Wei W., Ye W., Li X., Zhao W., Yang C. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019;5:5. doi: 10.1038/s41421-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hu Z.F., Gu A.D., Liang L., Li Y., Gong T., Chen J.J. Construction and optimization of microbial cell factories for sustainable production of bioactive dammarenediol-II glucosides. Green Chem. 2019;21:3286–3299. [Google Scholar]
- 226.Liang H., Hu Z., Zhang T., Gong T., Chen J., Zhu P. Production of a bioactive unnatural ginsenoside by metabolically engineered yeasts based on a new UDP-glycosyltransferase from Bacillus subtilis. Metab Eng. 2017;44:60–69. doi: 10.1016/j.ymben.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 227.Gutmann A., Bungaruang L., Weber H., Leypold M., Breinbauer R., Nidetzky B. Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions. Green Chem. 2014;16:4417–4425. [Google Scholar]
- 228.Chen L., Sun P., Zhou F., Li Y., Chen K., Jia H. Synthesis of rebaudioside D, using glycosyltransferase UGTSL2 and in situ UDP-glucose regeneration. Food Chem. 2018;259:286–291. doi: 10.1016/j.foodchem.2018.03.126. [DOI] [PubMed] [Google Scholar]
- 229.Dai L., Liu C., Li J., Dong C., Yang J., Dai Z. One-pot synthesis of ginsenoside Rh2 and bioactive unnatural ginsenoside by coupling promiscuous glycosyltransferase from Bacillus subtilis 168 to sucrose synthase. J Agric Food Chem. 2018;66:2830–2837. doi: 10.1021/acs.jafc.8b00597. [DOI] [PubMed] [Google Scholar]
- 230.Eibl R., Meier P., Stutz I., Schildberger D., Hühn T., Eibl D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl Microbiol Biotechnol. 2018;102:8661–8675. doi: 10.1007/s00253-018-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]