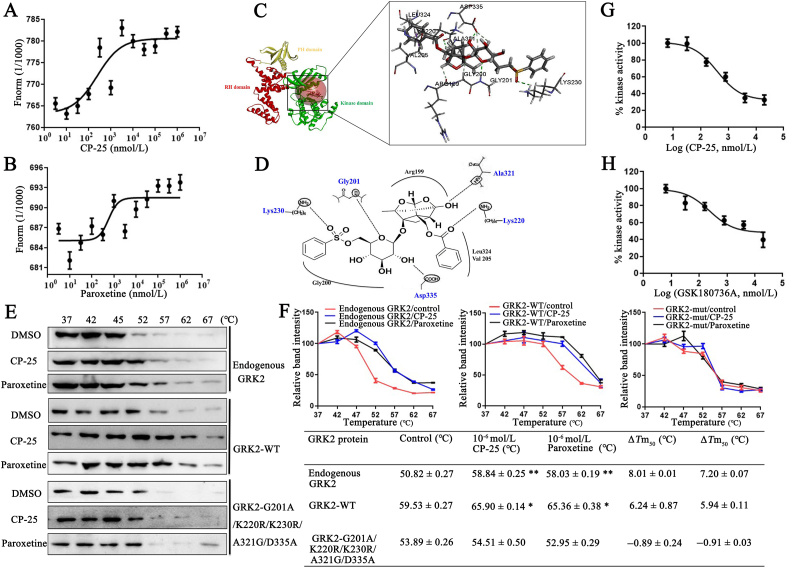
Figure 6.
CP-25 directly binds to the kinase domain of GRK2 and inhibits GRK2 activity. (A) and (B) Microscale thermophoresis (MST) of CP-25 and paroxetine. GRK2 (0.49 mg/mL) were incubated with increasing concentrations of CP-25 (A) and paroxetine (B). The interactions of CP-25 or paroxetine and GRK2 were quantified by MST and binding data were plotted applying the Kd equation. (C) Molecular docking modeling of compound CP-25 and GRK2, the small molecule and the critical interaction of 3KRW are represented by sticks. Panel is a view into the active site cavity. (D) Schematic representation of the binding mode of CP-25 in the GRK2 binding site of 3KRW. (E) Cellular thermal shift assay (CETSA) presented the thermal stability of endogenous GRK2, wild-type GRK2 (GRK2-WT), and GRK2 G201A/A321G/D335A/K220R/K230R mutant proteins under treatment with CP-25 (10−6 mol/L) and paroxetine (10−6 mol/L). (F) CETSA curve and the thermal stability to reach 50% of temperature (Tm50) value was performed using GraphPad Prism software. Data are expressed as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 vs. control group. (G) and (H) Determining IC50 for CP-25 and GRK2 inhibitor. IC50 were determined using the ADP-Glo™ Kinase Assay. Curve fitting was performed using GraphPad Prism software. All data are expressed as mean ± SD.