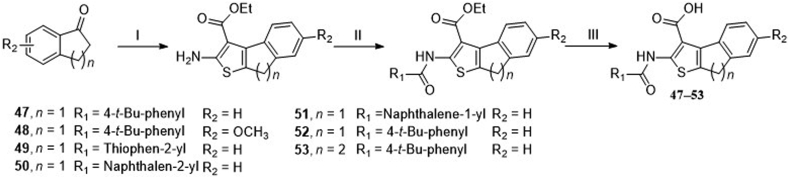
Scheme 2.
Synthetic route of 8H-indeno [2,1-b] thiophene-3-carboxylic acid and 4, 5-dihydronaphtho [2,1-b] thiophene-1-carboxylic acid derivatives.. Reagents and conditions: (I) step 1: ethyl cyanoacetate, ammonium acetate, toluene, acetic acid, reflux, 12 h, argon; step 2: sulfur, ethanol, argon, 60 °C, 36–48 h. (II) Aryl substituted acid chloride, TEA, DCM, rt, 8 h. (III) NaOH, H2O, MeOH, THF, 60 °C, 8 h.