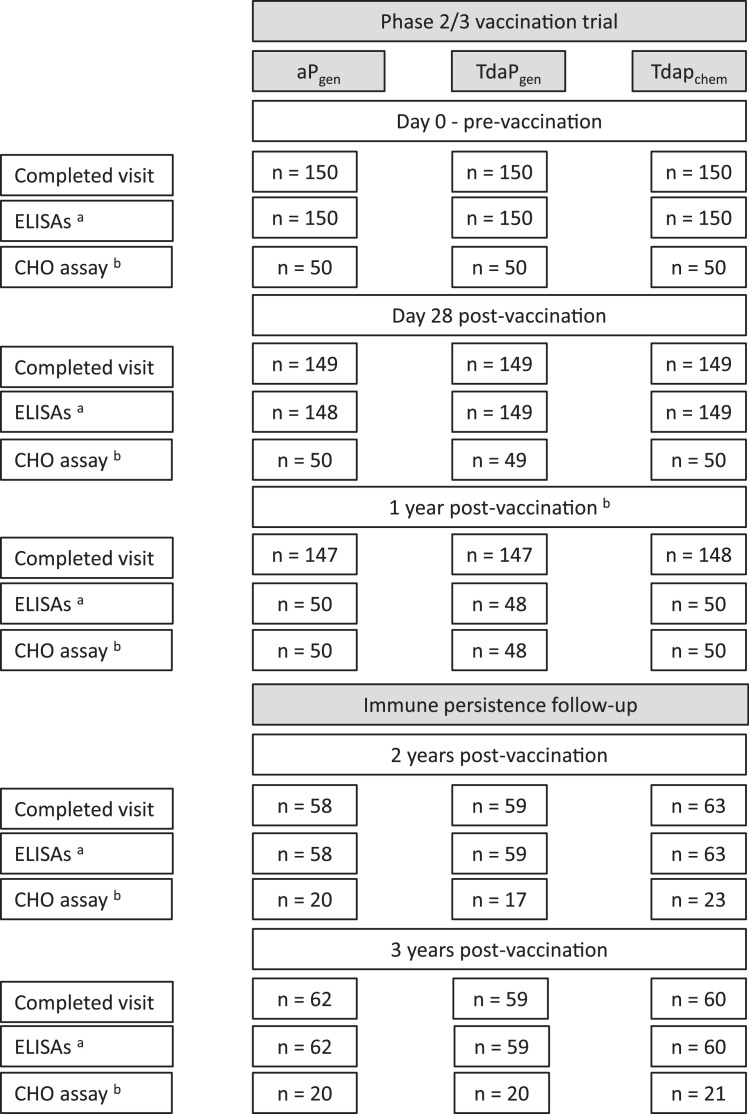
Fig. 1.
Subject disposition for initial trial and extended follow-up studies.
Provided are the number of participants who completed study visits and the number of blood samples analysed for IgG responses against pertussis antigens (PT and FHA) and diphtheria and tetanus toxoids as assessed by ELISA (a), and PT-neutralising antibody titres as assessed by the CHO neutralisation assay (b) as part of the initial trial (pre-vaccination, and Day 28 and 1-year post-vaccination) and the follow-up studies (2-year and 3-year post-vaccination).