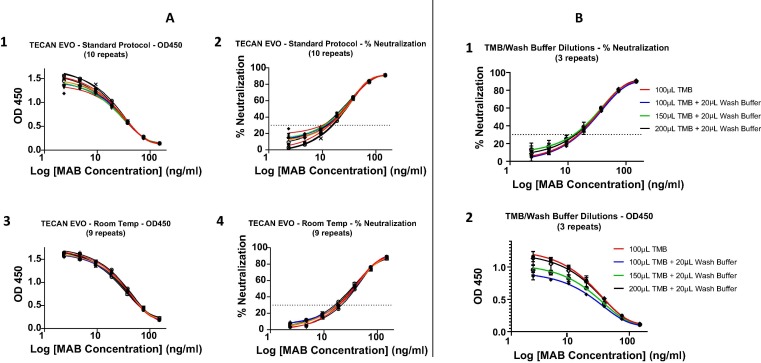
Fig. 1.
Optimization of cPass sVNT for liquid handler automation. A. Ambient temperature incubations. Ten replicates of a standard curve using the kit insert (KI) “Standard Protocol” were compared to nine replicates from an optimized ambient temperature “Room Temp” procedure (see section 2.5) using a well-characterized neutralizing monoclonal antibody (MAB) (GenScript #A02051) blocking the RBD-ACE2 interaction. The MAB was diluted serially by a factor of 1:2 from 150 ng/mL down each column of the plates to assess and compare intra-plate variability. The data between the two procedures were compared by plotting both the raw (OD450) values (A1 and A3) and the transformed % neutralization results (A2 and A4) vs Log of Ab Concentration. Within the linear range (ie: the second and third MAB dilutions) the difference between the standard (manual) and Tecan (automated) protocols was within 20% CV. B. Effect and compensation of wash buffer dilution of TMB. Mixtures of 20 µL residual wash buffer with the KI recommended 100 µL as well as 150 µL and 200 µL TMB were compared with the KI protocol of no residual wash buffer with 100 µL TMB. The data between the two procedures were compared by plotting both the raw (OD450) values (B2) and the transformed % neutralization results (B1) vs Log of Ab Concentration. Using a 2-way, multiple comparisons ANOVA test, a statistically significant difference was revealed within the linear range between the baseline (100 µL TMB) and the other conditions that included wash buffer with the exception of “20 µL wash buffer + 200 µL TMB” where there was no statistically significant difference.