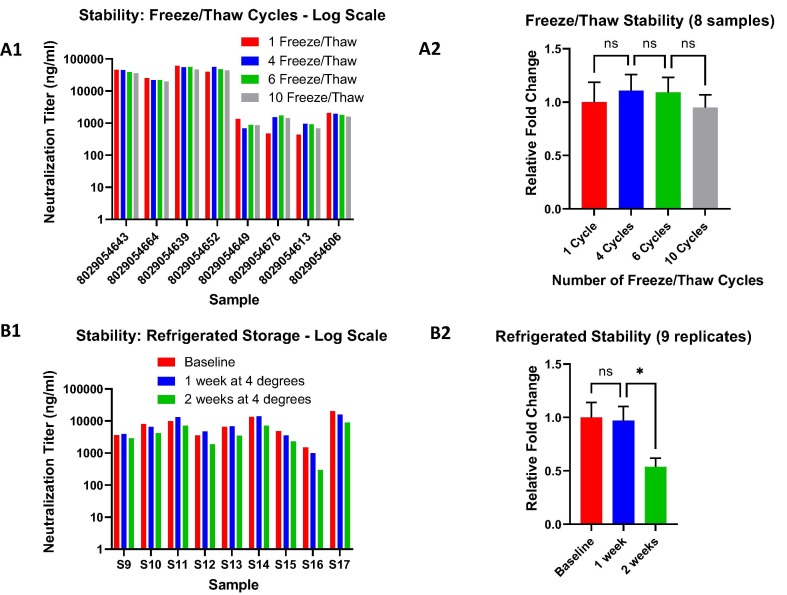
Fig. 6.
Stability testing of immune response antibody activity. A. Freeze/Thaw cycles. Up to 10 freeze/thaw cycles were assessed for samples with high and low neutralization antibody titers (A1) with no statistically significant difference between them as measured with a one way ANOVA (multiple comparisons) test on the combined, normalized sample data (A2). B. Refrigerated storage. Samples stored for one and two weeks at 4°were tested (B1). Although, there was a significant difference between the baseline/1 week and the 2 week refrigerated cycles the decrease in titers were only about two-fold (B2). For both refrigerated and frozen stability, all samples were tested semi-quantitatively as described in Fig. 3. ns: non-significant difference; * statistically significant (p-value = 0.0451).