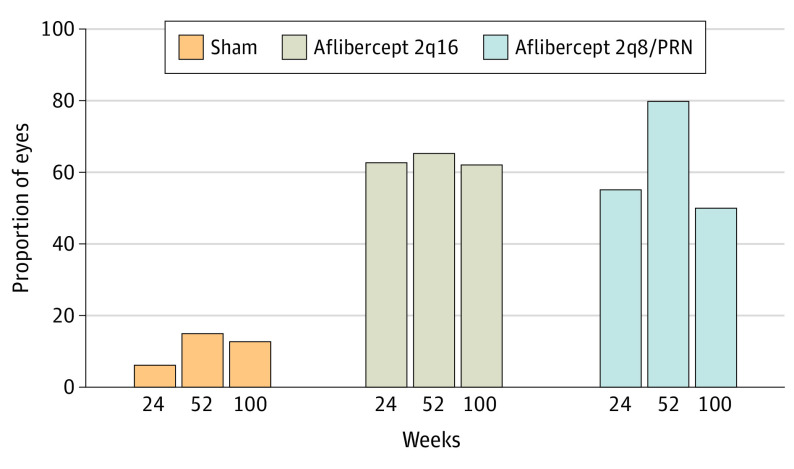
Figure 2. Proportion of Eyes With a 2-Step or Greater Improvement on Diabetic Retinopathy Severity Scale From Baseline Through Week 100.
Eyes in the aflibercept 2q16 group (n = 135) received intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval; eyes in the aflibercept 2q8/PRN group (n = 134) received intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses, with pro re nata (PRN) dosing beginning at week 56; and eyes in the control group (n = 133) received sham injections. The full analysis set was analyzed using the last observation carried forward method. At week 52, P < .001 for aflibercept 2q16 vs sham (65.2% vs 15.0%) and aflibercept 2q8/PRN vs sham. At week 100, P values for aflibercept 2q16 vs sham and aflibercept 2q8/PRN vs sham were nominal.