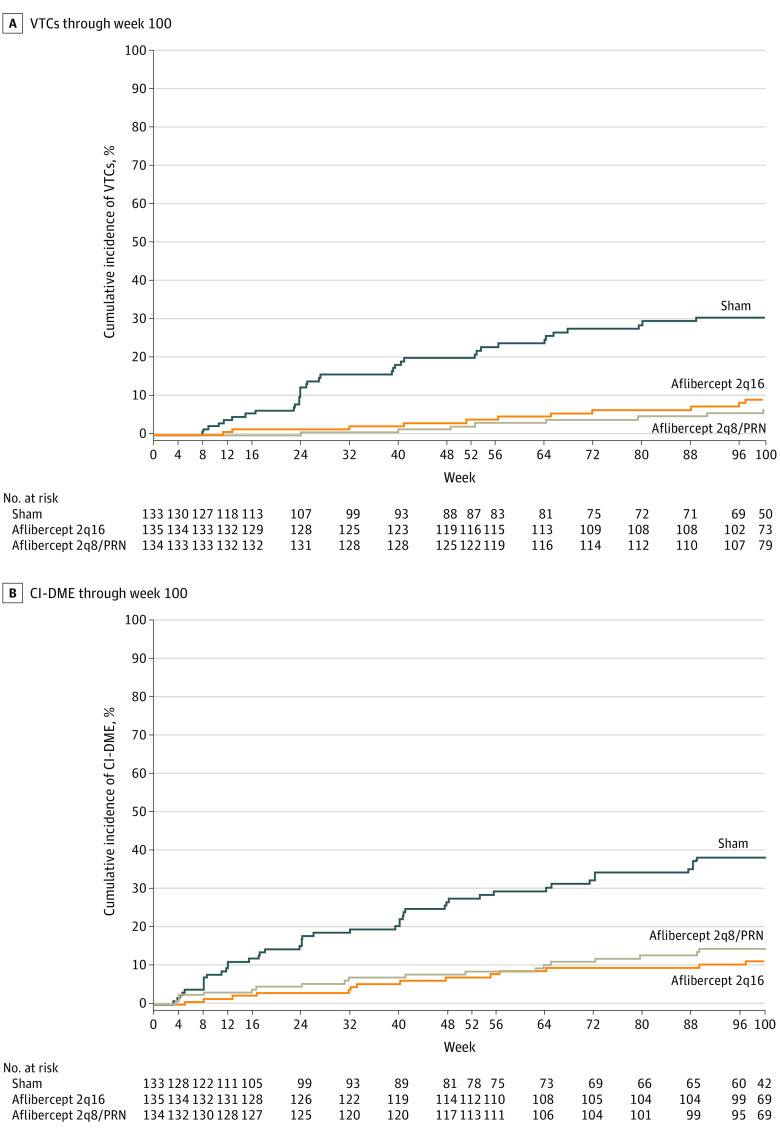
Figure 4. Cumulative Incidence of Events Indicative of Diabetic Retinopathy Progression.
Eyes in the aflibercept 2q16 group (n = 135) received intravitreal injections of aflibercept, 2 mg, every 16 weeks after 3 initial monthly doses and one 8-week interval; eyes in the aflibercept 2q8/PRN group (n = 134) received intravitreal injections of aflibercept, 2 mg, every 8 weeks after 5 initial monthly doses, with pro re nata (PRN) dosing beginning at week 56; and eyes in the control group (n = 133) received sham injections. A, VTCs through week 100. The cumulative event rate at week 100 was 9.1% in the aflibercept 2q16 group (hazard ratio [HR], 0.23; P < .001) and 6.9% in the aflibercept 2q8/PRN group (HR, 0.17; P < .001) compared with 30.6% in the control group. B, CI-DME through week 100. The cumulative event rate at week 100 was 11.3% in the aflibercept 2q16 group (HR, 0.24; P < .001) and 14.4% in the aflibercept 2q8/PRN group (HR, 0.32; P < .001) compared with 38.4% in the control group. CI-DME indicates center-involved diabetic macular edema (diagnosed by the investigator); VTC, vision-threatening complication (including proliferative diabetic retinopathy and/or anterior segment neovascularization diagnosed by the investigator and/or the reading center).