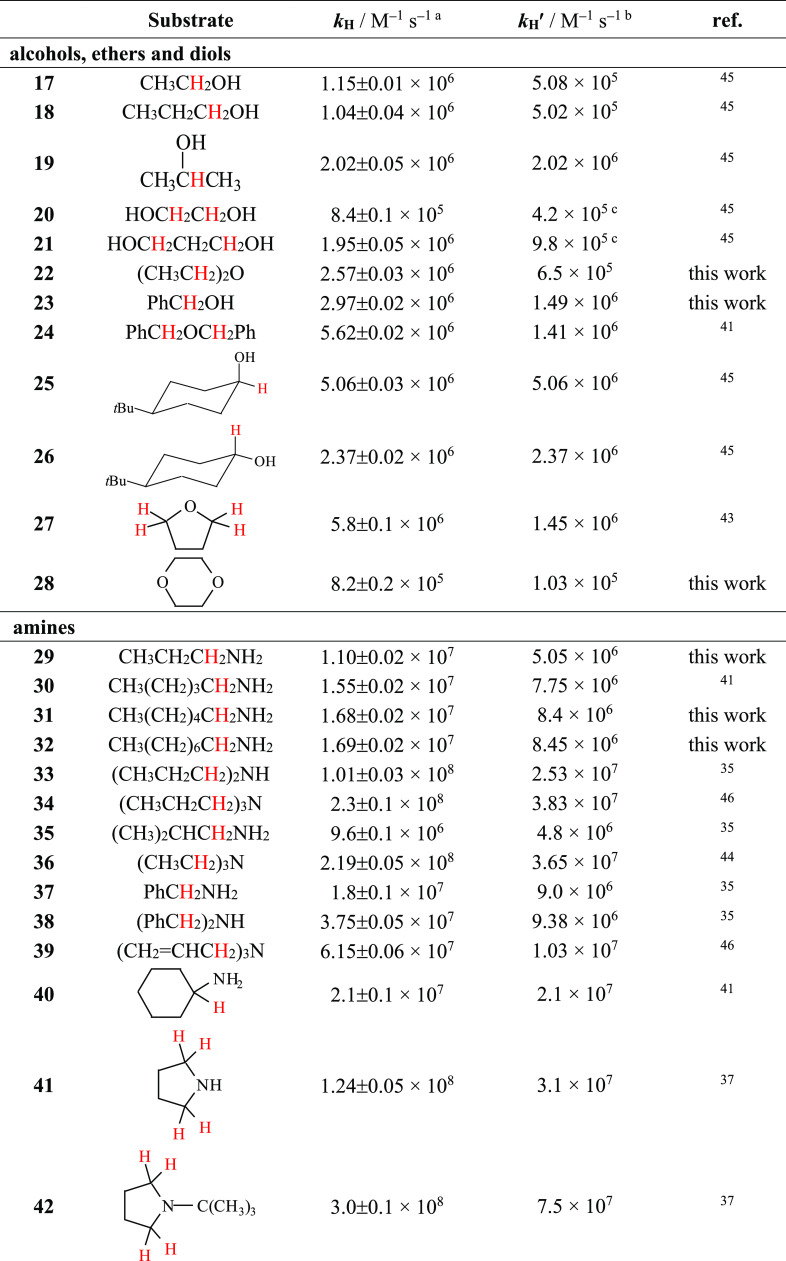
Table 2. Second-Order Rate Constants (kH) for Reaction of the Cumyloxyl Radical (CumO•) with Different Substrates.
Measured in Ar- or N2-saturated MeCN solution at T = 25 °C by 355 nm LFP, [dicumyl peroxide] = 1.0 M. kH values were determined from the slope of the kobs vs [substrate] plots, where in turn kobs values were measured following the decay of the CumO• visible absorption band at 490 nm. Average of at least two determinations.
kH′ = kH/n, where n represents the number of equivalent abstractable hydrogen atoms.
Calculated considering n = 2 based on the difference between the C–H BDEs of the two methylene groups in an intramolecular hydrogen-bonded structure (see text).