Abstract
Background:
We aimed to demonstrate the safety and efficacy of bronchial artery embolization (BAE) in patients with pulmonary tuberculosis in the planned management of “mild” hemoptysis. This treatment, already widely documented and used as a life-saving therapy in an emergency regimen, if properly planned in poorly controlled patients through medical therapy alone, can provide a valid opportunity by reducing the frequency and extent of non-fatal bleeding, but which still worsen the quality of life of these already significantly traumatized patients.
Methods:
All procedures were conducted through a right common femoral access with a 5 Fr catheter and a 2.7 Fr super-selective catheter coaxial technique of the branches of the bronchial arteries with suspected bleeding sources. Embolizations were performed with 500-700 micron Terumo PVA plastic microparticles. We decided to adopt the following inclusion criteria for the selection of patients to be enrolled: documented diagnosis of pulmonary TB, the presence of at least one bleeding episode that required at least two blood transfusions, evaluation with bronchoscopic examination to ascertain the bronchial origin of bleeding and the affected lobar site, execution of an angio-ct radiological study for the evaluation of the bronchial systemic anatomy as well as the patency of the pulmonary arterial circulation, general hemodynamic compensation and an age of enrollment between 25 and 65 years.
Results:
All selective embolization interventions demonstrated a technical success of 100% of the total number of patients. 11 out of 12 patients did not show any signs of relapse or complications related to the interventional procedure at a first check-up carried out at 48 hours, instead a fatal massive hemoptysis occurred in only one patient. At the next three-month follow-up, no relapses were documented in all selected patients. Only one patient required a second embolization four months after the first procedure.
Conclusions:
Radiological-interventional approach in the elective regimen of super-selective embolization of the bronchial arteries (BAE) in the management and control of “mild” hemoptysis in patients with pulmonary tuberculosis not controlled exclusively by medical therapy, according to a strategy systematic of planned intervention and respecting clear and standardized inclusion criteria, represented in our experience a safe and effective procedure, free from significant short and long term complications, especially in well selected patients, which, although not always allows a definitive and stable control of hemoptysis, can in any case significantly limit the risks, also allowing a better planning of the most appropriate therapeutic intervention strategy.
Keywords: hemoptysis, embolization, bronchial artery, endovascular, interventional, pulmonary tuberculosis
Introduction
Hemoptysis, or the expectoration of blood from the respiratory tract and/or lung parenchyma, is a clinical sign common to various diseases. There are specific parameters that allow the patient to be included in a therapeutic program according to the severity and general clinical conditions. Hemoptysis, especially if recurrent, requires a thorough investigation and a prompt treatment.
The bronchial arteries are vessels of the systemic circulation that usually originate at the level of the descending thoracic aorta at the D5-D6 level (1).
Bronchial arteries that do not originate at the level of D5-D6 are considered aberrant and may instead originate from the aortic arch, the brachiocephalic trunk, the subclavian artery, the mammary artery, but also from the phrenic artery or the abdominal aorta (2).
Generally the bronchial arteries not only vascularize the trachea and the bronchial tree, but also the esophagus, the visceral pleura and the loco-regional lymph nodes, and have a diameter of about 1.5 mm and a rectilinear course receiving about 1 % of cardiac output (3). In pathological conditions they show an increased diameter (> 2 mm), a tortuous course and can collect, in the most serious situations, up to 30% of the systolic range (4,5).
The embolization of the bronchial arteries to control massive hemoptysis is a technique already widely used as an emergency procedure (6); to date, the most common indications are represented by massive hemoptysis in the course of tuberculosis and in the outcomes of disease including bronchiectasis and cystic fibrosis (7,8). Another indication reported in the literature is the management of complications of AVM and arteriovenous fistulas in Rendu-Osler-Weber disease (HHT). It is difficult to define the extent of a hemorrhage quantitatively only on the basis of the volume of blood lost, especially since there are no standardized absolute values in the literature (9,10). According to the guidelines proposed by the SIP/IRS in accordance with AIPO, in the context of active bleeding in the course of a chronic non-traumatic disease, a “small” hemoptysis is defined as a blood loss of less than 5 ml, while a bleeding is defined as “mild” when is greater than 5ml. Finally, “massive” hemoptysis means bleeding greater than 240 ml per single episode or a sputum of 300-600 ml/24h. In the event of massive bleeding, the need for an emergency embolization procedure is obvious.
Methods
It is clear that the “small” hemoptysis does not require hospitalization, nor particular therapeutic measures, if not the observation and suspension of NSAIDs if taken. “Massive” hemoptysis, on the other hand, requires urgent selective embolization of the branches of the bronchial systemic circulation, responsible for bleeding, as the last life-saving procedure for the patient when the clinical conditions are unstable (11,12).
Therapeutic options, on the other hand, as regards the management of patients with “mild” hemoptysis are not very clear from what is learned in the literature, as if on the one hand medical therapy alone is recommended as a support tool through hospitalization and observation, incidence of further relapses are estimated at around 70% of cases (13,14).
The latter ones are conditioned by the persistence of specific risk factors and by the individual predisposition to bleeding (15). The inadequate control of episodes of “light” bleeding, even in hospitalized patients, can predispose in a high percentage of patients to a massive hemoptysis treatable this time exclusively in urgency through embolization, as the only life-saving intervention, without the possibility of adequate preparation of the patient and without having agreed on the best therapeutic planning of the case, in conditions in which the bleeding itself can be difficult to control since the pressures in the systemic bronchial arteries are higher than those present in the pulmonary circulation (16,17).
In most patients, death occurs due to asphyxiation from aspirated blood, while only a modest percentage is caused by the shock resulting from hypotension from haemorrhage (18). This implies that recurrent episodes of light bleeding, while on the one hand do not significantly alter the patient’s volume and hemodynamic status, on the other hand increase the risk of a subsequent fatal massive bleeding. Determining the hemorrhage site is the first step through appropriate radiological and bronchoscopic examinations to decide the correct intervention strategy (19).
The rationale for embolizing hemoptysis therapy is a very complex problem. Thanks to the implementation of new tools and materials, it was also possible to significantly reduce the risk of peri-procedural complications. The nature of the underlying disease and the underlying individual predisposition, particularly age, were important factors to consider in the treatment decision. In the period between January 2017 and September 2019, 12 procedures for embolization of bronchial arteries were successfully performed in our interventional radiology service in patients with pulmonary tuberculosis who met all the inclusion criteria adopted (20,21). (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig 5, Fig. 6). Inclusion criteria adopted for the selection of patients to be enrolled are shown in Table 1.
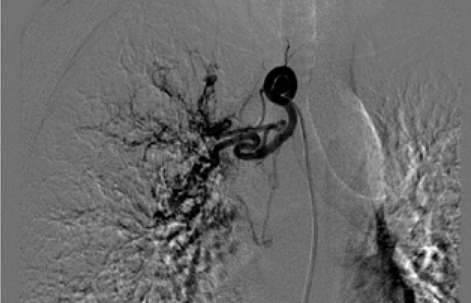
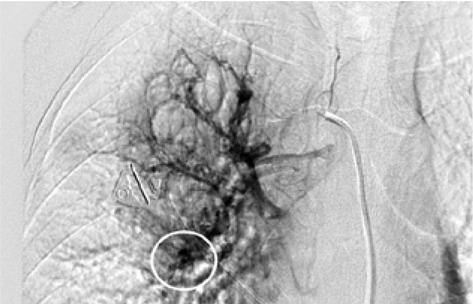
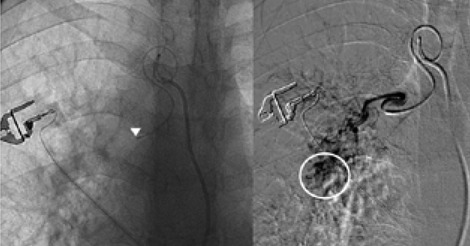
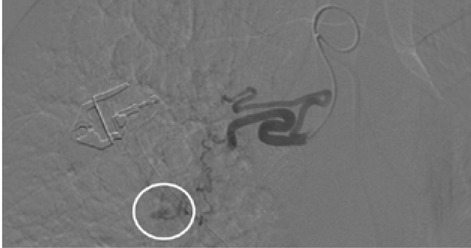
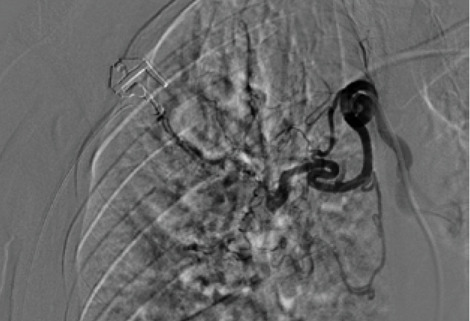
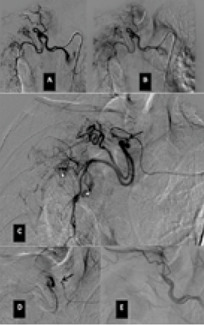
Table 1.
Inclusion criteria adopted for the selection of patients to be enrolled
| Documented diagnosis of pulmonary TB. |
| Presence of at least one bleeding episode that required at least two blood transfusions. |
| Evaluation with bronchoscopic examination to ascertain the bronchial origin of bleeding and the affected lobar site. |
| Execution of an angio-ct radiological study for the evaluation of the bronchial systemic anatomy as well as the patency of the pulmonary arterial circulation. |
| General hemodynamic compensation. |
| Age of enrollment between 25 and 65 year. |
Results
All selective embolization interventions demonstrated a technical success of 100% of the total number of patients. A first evaluation was performed 48 hours after the procedure, while a close follow-up was conducted at 3-6-12 months for the monitoring after some time. The criteria adopted for risk stratification after therapy were the monitoring of hemoglobin, the number and extent of relapses when they occurred and procedural complications over time. 11 out of 12 patients did not show any signs of relapse or complications related to the interventional procedure at a first check-up carried out at 48 hours, instead a fatal massive hemoptysis occurred in only one patient. Therefore, no peri-procedural complications or bleeding relapses over a short period of time were observed in 11 of 12 patients. At the next three-month follow-up, no relapses were documented in all selected patients. After six months, 2 patients continued not to witness any recurrence episodes, while 8 patients were documented with relapses of bleeding which however did not lead to a significant decrease in hemoglobin and therefore did not require any transfusion. On the other hand, only one patient required a second embolization four months after the first procedure. In this same patient, due to the technical difficulties in bronchial arterial catheterization, a more proximal embolization was primarily performed than the others (Table 2).
Table 2.
Statistics of the 12 patients enrolled with estimation of the risk of recurrences and therapeutic success.
| Technical success | 100% of the total number of patients (12/12). No peri-procedural complications. |
| First evaluation performed 48 hours after the procedure | No bleeding relapses in 11 of 12 patients (91,6%). Fatal massive hemoptysis in only one patient (8,3%). |
| Next three-month follow-up | No relapses on the remaining patients enrolled (11/11). |
| Next six-month follow-up | 1 only patient required a second embolization four months after the first procedure (9,09%). 2 patients didn’t show any recurrence episodes (18,18%). 8 patients documented with relapses of bleeding did not require any trasfusion (72,73%). |
Discussion
The estimate of bleeding cannot disregard the preliminary bronchoscopic evaluation and the angio-ct study of the selected patients. These two combined methods have been shown to have a high degree of sensitivity and specificity in confirming the bronchial origin as well as in demonstrating its exact lobar or segmental origin (22, 23, 24). A good prognosis cannot be separated from an accurate technical intervention planning and this in turn cannot be separated from an adequate and complete diagnostic evaluation of the bleeding in progress. In this way it was possible to achieve a high degree of procedural technical success, thanks to the identification, through the two combined methods, of findings to be attributed to active blood loss or to suspected sources of bronchial bleeding. In fact, we were able to discriminate on the basis of these evaluations both direct signs, such as the appreciable contrast blush during or shortly after the injection of contrast medium, and indirect signs of disease consisting in the hypertrophy and tortuosity of the vessels and in the evidence of arterio-arterial and arterio-venous broncho-pulmonary shunts. Thanks to these preliminary assessments, we were able to implement our technical skills thanks to an increase in our confidence with the anatomy of the bronchial circulation and with loco-regional hemodynamics. This result would have been difficult to achieve by excluding the combined instrumental evaluation from the diagnostic process. What has frequently been observed in previous years has been a progression of the underlying disease and a greater number of relapses often caused by incomplete embolization or recanalization of embolized systemic arteries. These latter complications have been greatly reduced through instrumental implementation and the more frequent use of super-selective catheterization. The super-selective approach has also allowed the catheterization of the bronchial arterial branches of smaller calibre, the most tortuous and distal ones, guaranteeing at the same time a better control of bleeding and a reduction of possible complications from embolization of systemic non-bronchial arteries such as arteries internal mammary artery, intercostal arteries and anterior spinal artery with non-negligible risks such as neurological complications due to spinal cord ischemia with transient or permanent deficits (25). The most frequent way of approaching the bronchial circulation, both from what we learn in the literature and from our experience, today appears to be through catheterization of the common femoral artery, however some authors have used in the past other approaches such as the transaxillary and trans -branchial, the latter in specific situations of need in which the anatomy of the aorta did not favor navigation of the catheter (26). In our study, the peri-procedural technical success of 100% of the total patients was favored by the use of a catheterization of no more than 5 Fr via the right common femoral access, with a 2.7 Fr microcatheter and coaxial technique that allowed almost all of cases and despite the interindividual anatomical variables of reaching the most distal bronchial arterial branches. Compliance and speed of intervention improved throughout the procedures. From our experience, there have been no complications related to catheterization using catheters not exceeding 5 Fr. Some patients, however, have had tussian reflexes upon injection of contrast medium, which in any case did not affect the success of the procedure or the prognosis. It remains to be considered whether this eventuality could still have repercussions on the bronchial mucosa in subsequent more in-depth evaluations. The choice of the same embolizing agents has evolved over the years, where polyvinyl alcohol (PVA) plastic particles have been widely used, singly or in association with other agents such as PVA hydrogels and microcoils. Today, the most commonly used embolizing agent is PVA with particles of size between 300 and 500 μm.
The PVA particles, available in different sizes, are not resorbable and act as a permanent occluding agent, however, the occlusion of the microcatheter in older works is reported in the literature as a disadvantage (27,28). In our study, embolizations were performed with 500-700 micron Terumo PVA plastic microparticles. According to our experience, the use of embolizing particles of at least 500 microns should be preferred and the use of particles smaller than 300 microns should be avoided because the chronic inflammatory state, typical of the primary pathology, determines a dimensional increase in broncho-pulmonary shunts, some of these already present even in physiological conditions. PVA plastic particles at least higher than 300 μm with a fair degree of confidence significantly limit the risk of pulmonary microembolism in other districts. In our opinion, liquid embolizers are also not recommended because they increase the risk of tissue necrosis and spirals due to the proximal occlusion which they determine by precluding a second embolization and poor control of the devices also with regard to micro-spirals (29,30). The spirals are instead currently used in aneurysms of the bronchial arteries. Despite the success of the embolization procedure on all patients and the absence of significant peri-procedural complications, we wondered about the possible causes that could determine the death due to asphyxia and internal drowning of the patient with fatal haemorrhage, reported at 48 hours from the procedure. During the night the patient developed a massive haemorrhage which resulted fatal to him which did not allow our interventional radiology service to be alerted in time, therefore it was not possible to carry out an emergency embolization quickly. The most probable cause of the event according to our considerations can be traced back to the immediate increase in bronchial arterial pressure generated in the bleeding area affected by the embolization, which subsequently determined an increase in blood flow in the previously unembolized branches since they were free from active extravasations, at the level of the same lobe or a different lobe, such as to cause a fatal haemorrhage.
Within 3 months of the embolization procedure, none of the other 11 patients developed hemorrhagic manifestations, significant drops in Hb that required blood transfusions, or other short-term complications. At the 6-month follow-up there was a second embolization operation, always performed in the elective regime, in a single patient who, 4 months after the first procedure, showed a quantitative and qualitative increase in bleeding episodes, not such to justify an intervention in urgency, but in any case deserving of a new investigation of the case through radiological and bronchoscopic examinations. The patient in question was the only one in whom we encountered procedural difficulties during the first embolization procedure, the latter dictated both by the district anatomical variability of the bronchial aterium tree and the navigation of the catheter, and by the technical compliance of the operators which increased during subsequent procedures. Indeed, in this specific case it was not possible to reach the most distal catheterization site possible, so we were forced to opt for a more proximal closure. The probable cause of re-bleeding, as well as in the intrinsic characteristics of the chronicity of the upstream disease, lies in the limited ability of the PVA particles to be able to guarantee a permanent closure of the embolized segment in case of proximal occlusions, since in this district the section of the vessel is greater and it is easy to understand how the risk of incomplete embolization and/or partial recanalization of the same after some time is not negligible.
The subsequent instrumental examinations have in fact localized the origin of the bleeding from the same afferent branch embolized previously during the first interventional approach. From this experience it emerges with more evidence that it is sufficient, at least necessary to aim at obtaining a super-selective catheterization in order to reach the most distal site of the vascular axis, therefore the one closest to the origin of the bleeding. Subsequently, we were able to carry out an evaluation at 12 months only in 7 out of 11 patients, since further considerations are still ongoing on the remaining part of the subjects, i.e. on the patients treated more recently on which it is not yet possible to express conclusive considerations. Of the 7 patients evaluated at one year, 2 patients who were already asymptomatic at six months continued to have no recurrence symptoms or reductions in hemoglobin, while of the other 5, who had minimal bleeding episodes at six months, 3 patients continued to experience sporadic bleeding episodes of the same magnitude, but slightly more than in the previous control at six months. In these last 3 patients a second integrated diagnostic angiotc + bronchoscopy study was therefore performed which established in 2 of the 3 patients the origin of the bleeding from a lobar branch other than the one affected by the embolization, while in the other patient the confirmed site was the same. All three patients did not undergo significant changes in hemoglobin, nor haemodynamic alterations and therefore did not require a second embolization. This result is explained by the very nature of the chronic pathology upstream of the haemorrhagic events. Since pulmonary tuberculosis is a chronic and in certain aspects evolutionary pathology, an embolization procedure at the level of the lung lobe or the segment affected by hemorrhage is able to stop the secondary event, but does not significantly affect the evolution of the lung disease (30).
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Informed consent:
Written informed consent was obtained from patient, and the study was approved by the ethics committee of the institution.
References
- Kalva S. P Bronchial Artery Embolization. Techniques in Vascular and Interventional Radiology. 2009;12:130–138. doi: 10.1053/j.tvir.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Butler J. Bronchial circulation: lung biology in healthand disease. 57:725–731. [Google Scholar]
- Cauldwell EW, Siekert RG, Lininger RE, Anson BJ. Thebronchial arteries: an anatomic study in 150 human cadavers. Surg Gynecol Obstet. 1948;86:395–412. [PubMed] [Google Scholar]
- Ananya panda, Ashu Seith Bhalla, Ankur Goyal. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. 2017:23307–317. doi: 10.5152/dir.2017.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevin Davidson, Samira Shojaee. Managing Massive Hemoptysis. Chest. 2020;157:77–88. doi: 10.1016/j.chest.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Fernando H C, Stein M, Benfield J R, Link D P. Role of bronchial artery embolization in the management of haemoptysis. Arch Surg. 1998;133:862–886. doi: 10.1001/archsurg.133.8.862. [DOI] [PubMed] [Google Scholar]
- Swanson K L, Johnson C M, Prakash U B, Mc Kusick M A, Andrews J C, Stanson A W. Bronchial artery embolization: experience with 54 patients. Chest. 2002;121:789–795. doi: 10.1378/chest.121.3.789. [DOI] [PubMed] [Google Scholar]
- Chun JY, Morgan R, Belli AM. Radiological management of haemoptysis: a comprehensive re-view of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240–250. doi: 10.1007/s00270-009-9788-z. [DOI] [PubMed] [Google Scholar]
- Sopko DR, Smith TP. Bronchial artery embolization for haemoptysis. Semin Interv Radiol. 2011;28:48–62. doi: 10.1055/s-0031-1273940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chan J W, Chan S C, et al. Bronchial artery embolisation can be equally safe and effective in the management of chronic recurrent haemoptysis. Hong Kong Med J. 2008;14:14–20. [PubMed] [Google Scholar]
- Haponik E F, Fein A, Chin R. Managing life-threatening haemoptysis: has anything really changed? Chest. 2000;118:1431–1435. doi: 10.1378/chest.118.5.1431. [DOI] [PubMed] [Google Scholar]
- Corvino A, Catelli A, Trovato P, et al. Volar ganglion cyst of the wrist simulating a radial artery pseudoaneursym: a case report. Embj. 2020;15(21):90–93. [Google Scholar]
- Hwang HG, Lee HS, Choi JS, et al. Risk factors influencing rebleeding after bronchial artery embolization on the management of hemoptysis associated with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2013;74:111–9. doi: 10.4046/trd.2013.74.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha C, Shyamkumar NK, Vinu M, et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Interv Radiol. 2012;18:96–101. doi: 10.4261/1305-3825.DIR.3876-11.2. [DOI] [PubMed] [Google Scholar]
- Kim Y G, Yoon H K, Ko G Y, Lim C M, Kim W D, Koh Y. Long-term effect of bronchial artery embolization in Korean patients with haemoptysis. Journal de Radiologie Diagnostique et Interventionnelle. 2015;96:333–346. [Google Scholar]
- Sopko DR, Smith TP. Bronchial artery embolization for haemoptysis. Semin Interv Radiol 2011. Semin Intervent Radiol. 2011;28(1):48–62. doi: 10.1055/s-0031-1273940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossi F, Maroldi R, Battaglia G, Pinotti G, Tassi G. Indicators predictive of success of embolisation: analysis of 88 patients with haemoptysis. Radiol Med. 2003;105:48–55. [PubMed] [Google Scholar]
- Hwang HG, Lee HS, Choi JS, Seo KH, Kim YH, Na JO. Risk factors influencing rebleeding after bronchial artery embolization on the management of hemoptysis associated with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2013;74(3):111–9. doi: 10.4046/trd.2013.74.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S, Ruttley M S. Bronchial artery embolization: the importance of preliminary thoracic aortography. Clin Radiol. 2000;55:317–319. doi: 10.1053/crad.1999.0084. [DOI] [PubMed] [Google Scholar]
- Drooz A T, Lewis C A, Allen T E, et al. Quality improvement guidelines for percutaneous transcatheter embolization. J Vasc Interv Radiol. 2003;14:237–242. [PubMed] [Google Scholar]
- Angle JF, Siddiqi NH, Wallace MJ, et al. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2010;21:1479–1486. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Ponnuswamy I, Sankaravadivelu ST, Maduraimuthu P, et al. 64-detector row CT evaluation of bronchial and non-bronchial systemic arteries in life-threatening haemoptysis. Br J Radiol. 2012;85:666–672. doi: 10.1259/bjr/24730002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza-Hernández CN, Ramírez-González JM, Cuéllar-Lozano RA, et al. Morphological Analysis of Bronchial Arteries and Variants with Computed Tomography Angiography. Biomed Res Int 2017. 2017:9785896. doi: 10.1155/2017/9785896. doi: 10.1155/2017/9785896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnuswamy I, Sankaravadivelu ST, Maduraimuthu P, et al. 64-detector row CT evaluation of bronchial and non-bronchial systemic arteries in life-threatening haemoptysis. Br J Radiol. 2012;85:666–672. doi: 10.1259/bjr/24730002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Yamakado K, Murashima S, et al. Super-selective bronchial artery embolization for haemoptysis with a coaxial microcatheter system. J Vasc Interv Radiol. 1997;8:65–70. doi: 10.1016/s1051-0443(97)70517-7. [DOI] [PubMed] [Google Scholar]
- Angle JF, Siddiqi NH, Wallace MJ, et al. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2010;21(10):1479–1486. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Woo S, Yoon CJ, Chung JW, et al. Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269:594–602. doi: 10.1148/radiol.13130046. [DOI] [PubMed] [Google Scholar]
- Cremaschi P, Nascimbene C, Vitulo P, et al. Therapeutic embolization of bronchial artery: a successful treatment in 209cases of relapse hemoptysis. Angiology. 1993;144:295–299. doi: 10.1177/000331979304400405. [DOI] [PubMed] [Google Scholar]
- Ryu YJ, Lee JH, Chun EM, et al. Clinical outcomes and prognostic factors in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis. 2011;15(2):246–50. [PubMed] [Google Scholar]
- Serebruany VL, Malinin AI, Eisert RM, et al. Risk of bleeding complications with antiplatelet agents: meta-analysis of 338, 191 patients enrolled in 50 randomized controlled trials. Am J Hematol 2004. 2004;75:40–7. doi: 10.1002/ajh.10451. [DOI] [PubMed] [Google Scholar]


