Fig. 2.
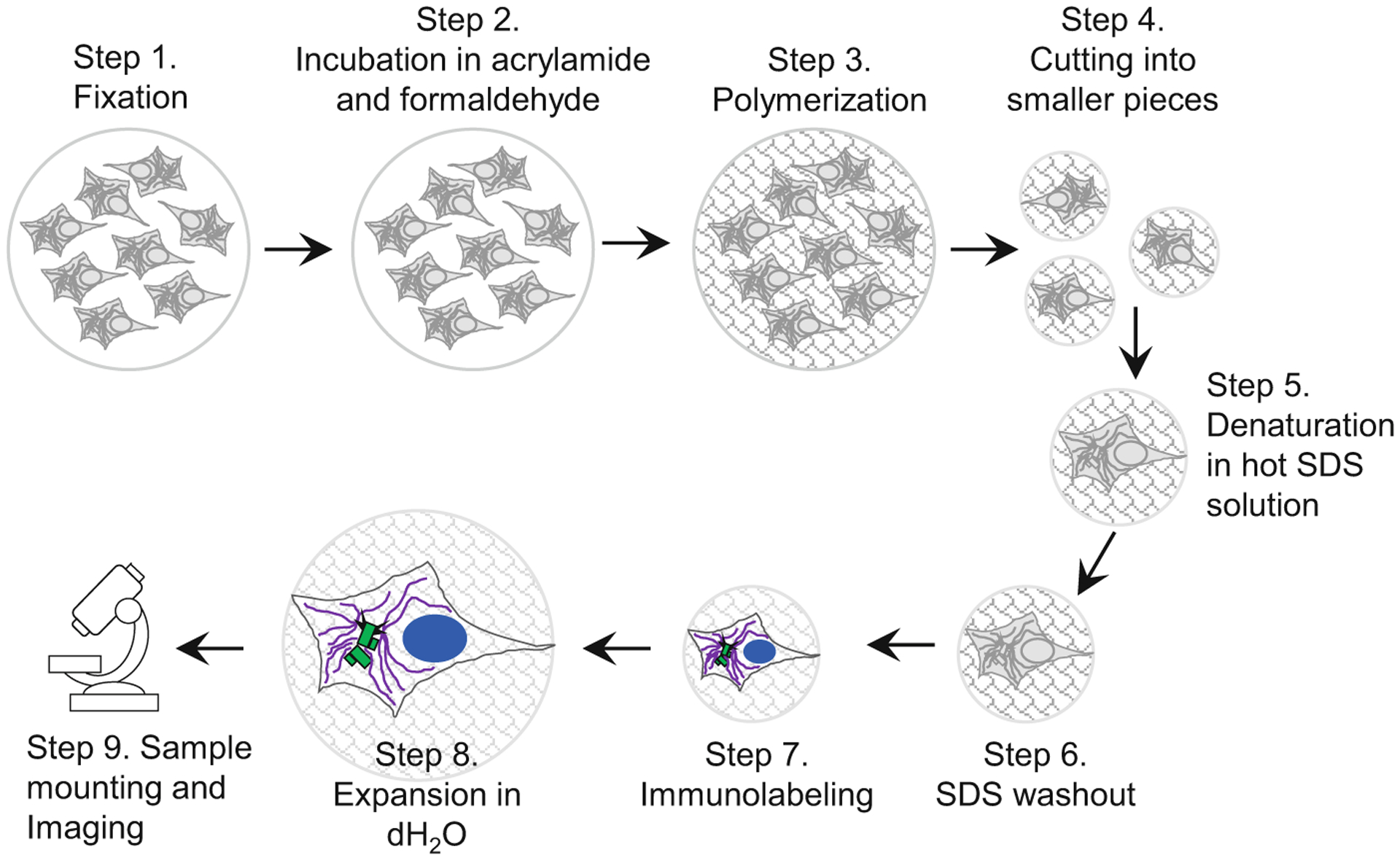
Key steps in sample preparation for expansion microscopy. A scheme illustrates major steps of the protocol, as described in the subheadings of Subheading 3. Cells growing on the coverslip are fixed and then incubated in a mixture of acrylamide and formaldehyde solution, which establishes chemical groups required for crosslinking of the sample components to the polymer during subsequent polymerization step. After polymerization, the sample is cut to several smaller samples. Individual samples (or ‘punches’ if a biopsy puncher is used to excise smaller pieces of the gel) are boiled in the presence of high SDS concentration, to denature proteins and to allow expansion of various cellular structures. During this time, the gel, and the biological specimen within, expands ~2-fold. In the subsequent step, SDS is thoroughly washed out of the gel, which is required for efficient immunolabeling. Proteins of interest are then immunolabeled with primary and secondary antibodies, and DNA is labeled using DAPI. After immunolabeling, samples are gradually expanded by incubation in dH2O
