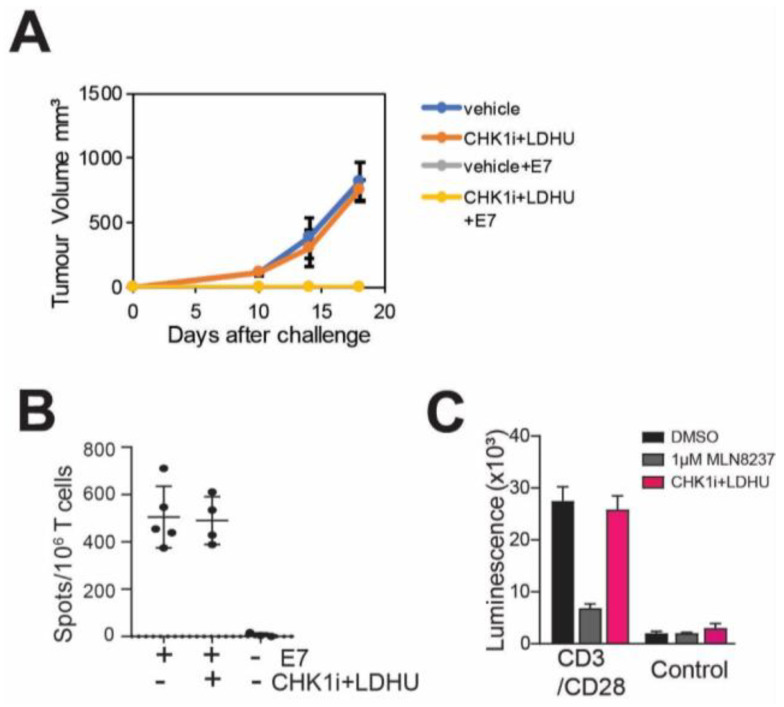
Figure 3.
CHK1i+LDHU does not adversely affect a T cell-mediated immune response. (A) Immune competent C57BL/6J mice were treated with either vehicle or the normal three-week regimen of CHK1i+LDHU. In the second week of treatment, mice were immunised or not with MHC class I-restricted HPV E7 (RAHYNIVTF) peptide, and one week after completion of the drug treatment mice were challenged with live HPV E7 expressing syngeneic TC-1 tumour cells. Tumour growth was used as a measure of immune rejection. (B) Splenocytes isolated from the indicated mice from the experiment shown in A were stimulated with E7 peptide and an ELISpot assay was performed for the production of IFNγ. (C) Human donor-derived PBMC were treated with anti-CD3/CD28 beads to stimulate T cell proliferation for three days, then treated wither either vehicle (DMSO), 1 mM Alisertib or 0.2 mM SRA737+0.1 mM HU (CHK1i+LDHU) for three days, the drugs removed, and cells allowed to proliferate a further three days. Cell viability was assayed using CellTiter-Glo. The experiment was performed in triplicate and is representative of two independent experiments.