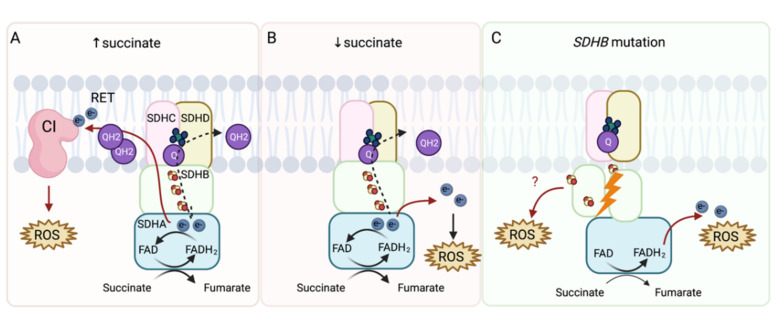
Figure 2.
ROS production from SDH under physiological and pathophysiological conditions. The SDHA subunit covalently binds flavin adenine dinucleotide (FAD), which removes electrons from succinate to form fumarate. SDHB then transfers electrons via three iron–sulfur (FeS) clusters to the ubiquinone molecule (Q) located in the ubiquinone-binding site, formed by the SDHB, SDHC, and SDHD subunit. Ubiquinone is further reduced to ubiquinol, fueling CIII and CIV (dashed arrow). (A) At high succinate concentrations (≥5 mM), succinate-derived electrons reduce the ubiquinone pool and electrons are forced backward to CI in a process called reverse electron transfer (RET), which leads to indirect ROS production. (B) At physiological succinate concentrations, ROS can be produced directly. FAD is reduced to FADH2, which then reacts with oxygen within an unoccupied binding site to form ROS. (C) In cases of SDHB mutation, incorrect assembly of SDH can result in ROS production directly from reactions between oxygen and FADH2 or exposed FeS clusters.