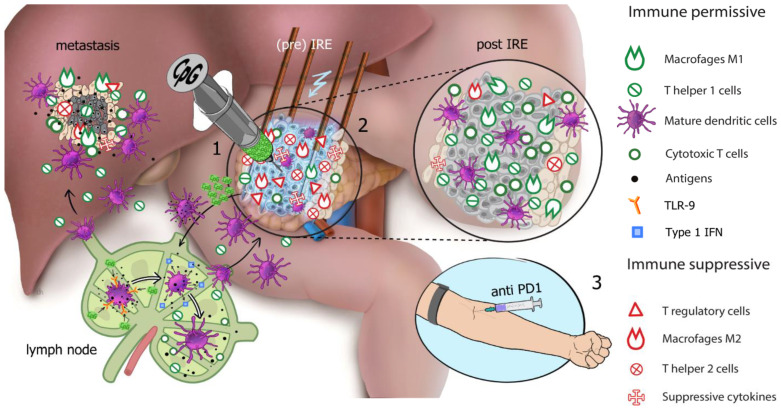
Figure 1.
Electroimmunotherapy. Illustrated is the proposed working mechanism of electroimmunotherapy and the three separate treatment stages to achieve synergistic immune modulation as performed in PANFIRE-III study arm C. The primary pancreatic tumor maintains an immunosuppressive microenvironment with the presence of regulatory T cells (Tregs), M2 Macrophages, T helper 2 cells, and suppressive cytokines (red icons), while the effector T cells (T helper 1 cells and cytotoxic T cells) are downregulated. Stage (1): Priming of the tumor microenvironment. CpG (cytosine-phosphate-guanine) type B oligodeoxynucleotide (CpG-B ODN) is injected into the primary tumor and diffuses to the primary tumor-draining lymph nodes where it binds to toll-like receptor 9 (orange receptor) on plasmacytoid dendritic cells (DCs), which mature and release type-1 IFN, which in turn can activate lymph node-resident conventional DCs (cDCs), causing these to mature (purple cells). Effective DC maturation results in their improved ability for tumor antigen uptake and presentation and stimulates type-1 IFN release (blue icons), resulting in the activation of cytotoxic- and helper T cells (green icons). Stage (2): Ablative antigen release and downregulation of tumor induced immune suppression. Irreversible electroporation (IRE) of the primary pancreatic tumor causes massive cell death resulting in antigen release (black dots). Antigens are taken up by DCs and transported back to the lymph nodes for T cell cross priming to result in adaptive tumor specific T cell responses (green icons). As IRE reduces the tumor mass, it reduces the secretion of immunosuppressive cytokines and consequently reduces numbers of circulating suppressive immune cell subsets (red icons). The tumor microenvironment shifts from immune suppressive (pre-IRE, red icons) to immune permissive (post-IRE, green icons). Stage (3): Enhancing the induced effector T cell response by intra venous injection of the anti-PD-1 monoclonal antibody (mAb). PD-L1 on the cancer cell surface binds to PD-1 on the T cell surface, inhibiting immune cell activity. Anti-PD-1 mAb (PD-1 checkpoint inhibitor) binds to the PD-1 receptors on the T cells, thereby blocking the receipt of inhibitory signals via PD-L1 and allowing the T cells to uninhibitedly attack the tumor cells (not illustrated). NB: new insights suggest that the PD-1 blockade can also release co-stimulatory signaling in T cells through CD28, leading to increased priming in tumor-draining lymph nodes. Thus, the combination of all three treatment modalities (IRE, CpG, Anti-PD-1) may work synergistically and together may alter the tumor microenvironment to induce a systemic immune response and ultimately cause an “anenestic” effect in distant metastatic lesions (illustrated in the liver). (Reprinted with permission of Geboers et al. [51].)