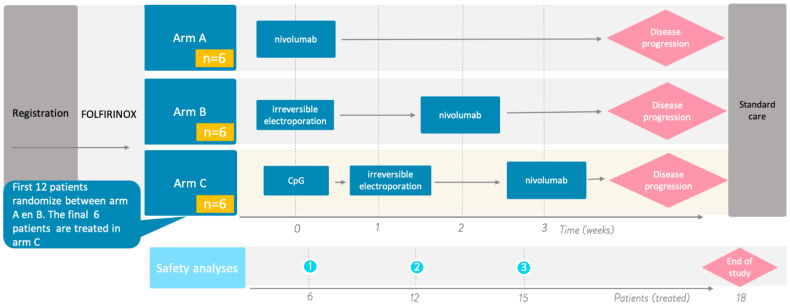
Figure 2.
PANFIRE III treatment schedule. All patients require pretreatment with a minimum of 8 cycles of FOLFIRINOX, with at least stable disease, prior to PANFIRE III inclusion. The first 12 patients will be randomized to treatment arm A or B. Arm C will open for the last 6 patients upon the validation of the interim safety analyses. These safety analyses will be performed after the treatment of 6, 12, and 15 patients. Arm A: nivolumab monotherapy. Arm B: irreversible electroporation succeeded by intravenous administration of nivolumab. Arm C: priming with intratumoral injection of CpG (IMO-2125), followed by irreversible electroporation after 1 week. This is succeeded by intravenous administration of nivolumab two weeks later. In all treatment arms, nivolumab will be administered until unequivocal disease progression.