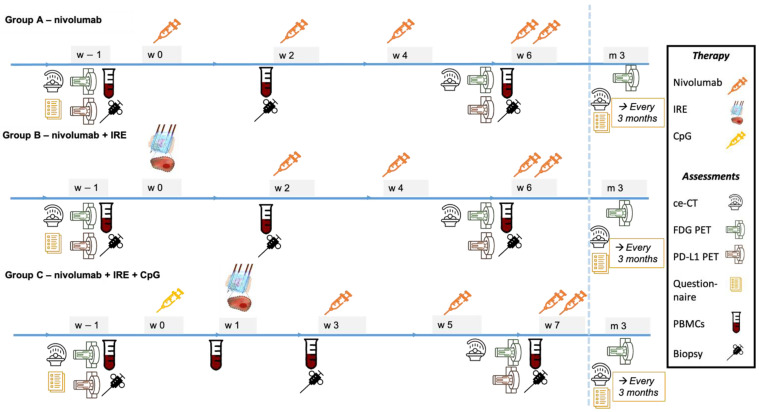
Figure 3.
PANFIRE III specified treatment schedule and study assessments per study arm. Arm A: nivolumab monotherapy; intravenous administration of 240 mg nivolumab every 2 weeks for 3 doses followed by intravenous administration of 480 mg nivolumab every 4 weeks. Assessments: ceCT (baseline, 5 weeks, and after every 3 months), FDG and PD-L1-PET (baseline and 5 weeks), blood and biopsy sampling (baseline, 2 weeks (prior to second nivolumab administration) and 5 weeks (prior to fourth nivolumab administration)), and QOL questionnaires (baseline and every 3 months). Arm B: irreversible electroporation succeeded by intravenous administration of 240 mg nivolumab every 2 weeks for 2 doses followed by intravenous administration of 480 mg nivolumab every 4 weeks. Assessments: ceCT (baseline, 5 weeks, and after every 3 months), FDG and PD-L1-PET (baseline and 6 weeks), blood and biopsy sampling (baseline, 2 weeks (prior to second nivolumab administration) and 5 weeks (prior to fourth nivolumab administration)), and QOL questionnaires (baseline and every 3 months). Arm C: priming with intratumoral injection of IMO-2125 after 1 week followed by irreversible electroporation. This is succeeded 2 weeks later by intravenous administration of 240 mg nivolumab every 2 weeks for 2 doses followed by intravenous administration of 480 mg nivolumab every 4 weeks. Assessments: ceCT (baseline, 6 weeks, and after every 3 months), FDG and PD-L1-PET (baseline and 6 weeks), blood and biopsy sampling (baseline, 1 week, 3 weeks (prior to first nivolumab administration), and 6 weeks (prior to third nivolumab administration)), and QOL questionnaires (baseline and every 3 months). In all treatment arms, nivolumab will be administered until unequivocal disease progression. W: week, M: month, IRE: irreversible electroporation, ceCT: contrast enhanced computed tomography, PET: positron emission tomography, FDG: fluorodeoxyglucose, PD-L1 PET: programmed death—ligand 1. Double syringe nivolumab at 6 (arm A/B) and 7 (arm C) weeks illustrates change of nivolumab dosing from 240 mg fortnightly to 480 mg monthly.