Figure 1.
Composition, productivity, and stability of AAVCOVID vaccine candidates
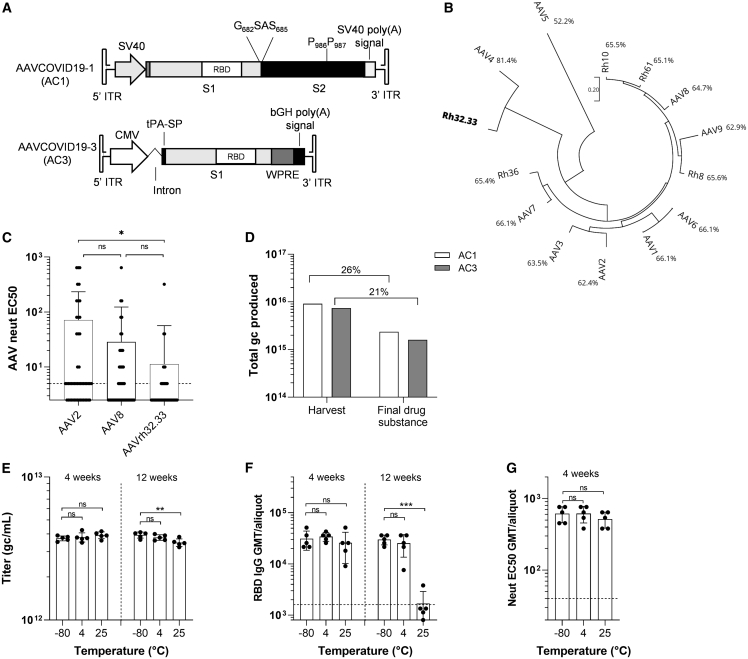
(A) Schematic representation of the recombinant genome of AAVCOVID19-1 (AC1) and AAVCOVID19-3 (AC3) vaccine candidates. SV40: Simian virus 40 promoter. RBD: receptor-binding domain. S1, S2: SARS-CoV-2 spike subunits 1 and 2. CMV: cytomegalovirus promoter. tPA-SP, tissue plasminogen activator signal peptide; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; bGH, bovine growth hormone; ITR, inverted terminal repeat.
(B) Phylogenetic tree of several AAV clades and percentage of sequence identity with AAVrh32.33.
(C) AAV2, AAV8, and AAVrh32.33 neutralizing antibody titer (EC50) in 50 human donor plasma samples.
(D) Total genome copies (gc) of AC1 and AC3 produced at large scale and quantified at harvest of producer cells and after the purification (final drug substance). Percentage of vector recovery during purification is displayed.
(E) Titer (gc/mL) of AC1 aliquots (n = 5) stored at −80°C, 4°C, and 25°C for 4 and 12 weeks.
(F) RBD-binding antibody titers in C57BL/6 animals 21 days after vaccination with 1011gc of AC1 aliquots (n = 5) stored at −80°C, 4°C, and 25°C for 4 and 12 weeks.
(G) Pseudovirus neutralizing titers (EC50) in C57BL/6 animals 21 days after vaccination with 1011gc of AC1 aliquots (n = 5) stored at −80°C, 4°C, and 25°C for 4 weeks.
(C and E–G) Data are represented as mean ± SD. One-way ANOVA and Tukey’s post-test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001
See also Figure S1.