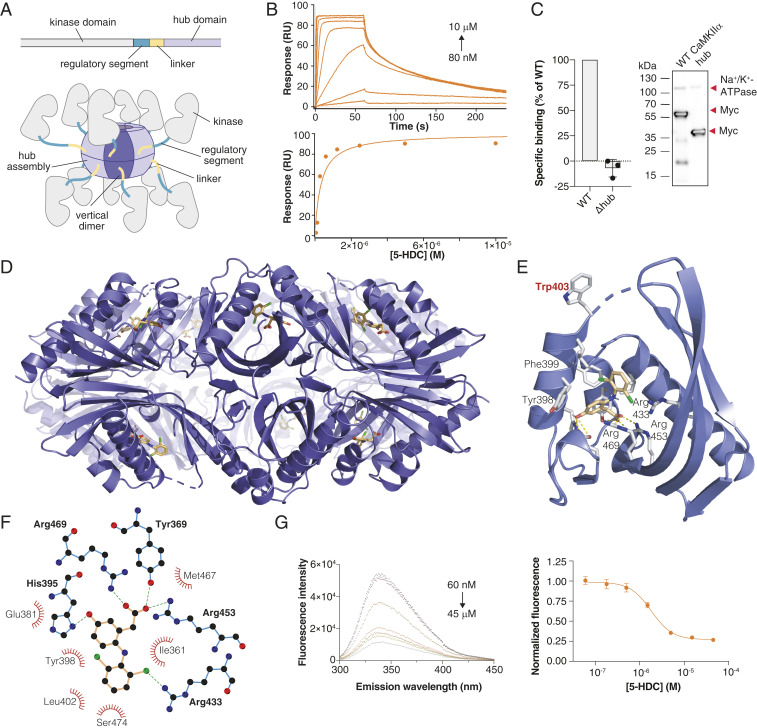
Fig. 2.
GHB analogs bind the CaMKIIα hub domain. (A) Schematic of a single CaMKIIα subunit composed of a kinase domain (gray), regulatory segment (green), linker (yellow), and hub domain (lilac). Twelve to 14 hub domains oligomerize into the holoenzyme, shown here in an activated form. (B) Concentration-dependent binding of 5-HDC to immobilized CaMKIIα 6x Hub measured by surface plasmon resonance (Top); Langmuir-binding isotherm (Bottom), representative data (see also SI Appendix, Fig. S4 B–F). (C) Absence of [3H]HOCPCA binding to the CaMKIIα mutant lacking the hub domain (Δhub) with representative Western blot showing expected sizes. Na+/K+-ATPase was used as a loading control; red arrows indicate the relevant bands. (D) X-ray crystal structure of 5-HDC bound to the CaMKIIα 6x Hub (14-mer). (E) Close-up view of a single hub subunit showing the key molecular interactions with displacement (flip) of Trp403 with ligand bound highlighted. (F) Ball and stick model of key binding residues (bold), nearby residues, and hydrogen bonds in green-dashed lines (for electron densities, reference SI Appendix, Fig. S5). (G) Quenching of intrinsic tryptophan fluorescence caused by Trp403 flip (6× Hub) with increasing concentrations of 5-HDC (Left) and resulting inhibition curve (Right) (n = 8), pooled data (means ± SEM) (see also SI Appendix, Fig. S6 A–C).