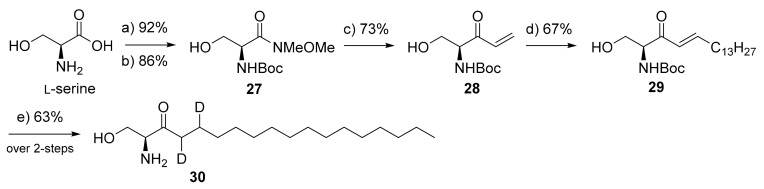
Scheme 5.
Synthesis of 3-ketosphinganine-d2 (30). Reagents and conditions: (a) Boc2O, 1 M NaOH, dioxane, −10 °C to r.t., 4 h; (b) Me(MeO)NH.HCl, EDCI.HCl, NMM, DCM, −15 °C, 2 h; (c) (i) n-BuLi, THF, −65 °C, 30 min, (ii) vinylmagnesium bromide (1 M in THF), −65 °C- r.t., 6 h; (d) 1-pentadecene, Grubbs 2nd generation cat.(7 mol%), DCM, 40 °C, 6 h; (e) (i) D2, 10% Pd/C, d-acetic acid (cat.), d-Methanol, r.t., 12 h, (ii) 4 M HCl, THF-MeOH, reflux, 2 h.