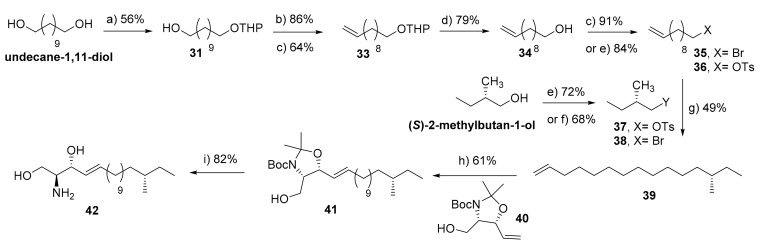
Scheme 6.
Synthesis of (16S)-methyl-sphingosine (42). Reagents and conditions: (a) DHP, PTSA (cat.), THF, 0 °C-r.t., 16 h; (b) CBr4, PPh3, DCM, 0 °C-r.t., 4 h; (c) t-BuOK, THF, reflux, 12 h; (d) PPTS (cat.), EtOH, 62 °C, 2 h; (e) TsCl, pyridine, DCM, 0 °C-r.t., 16 h; (f) NBS, PPh3, DCM, 0 °C-r.t., 2 h; (g) (i) 38, Mg, THF, 30 °C, 90 min, and then (ii) 43, Li2CuCl4 (cat), THF, −78 °C-r.t., 16 h; (h) 40, Grubbs 2nd generation (cat.), DCM, reflux, 12 h; (i) AcCl, MeOH, 0 °C-r.t., 2 h.