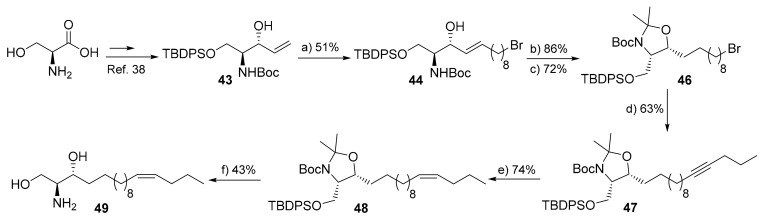
Scheme 7.
Synthesis of 14Z-sphingosine (49). Reagents and conditions: (a) 10-bromo-1-decene, p-benzoquinone (10 mol%), CuI (3 mol%), Grubbs 2nd generation cat. (10 mol%), d-chloroform, 35 °C, 14 h; (b) H2, 10% Pd/C, acetic acid (cat.), THF, r.t., 16 h; (c) 2,2-dimethoxypropane, p-TsOH (cat.), toluene, 80 °C, 3 h; (d) (i) 1-pentyne, tert-BuLi, THF, −78 °C, 2 h, (ii) 46, HMPA, THF, −78 °C- r.t.,14 h; (e) H2, Lindlar cat., EtOAc, DMF, r.t., 12 h; (f) (i) TBAF, THF, 55 °C, 3 h, (ii) AcCl, MeOH, 0 °C-r.t., 3 h.