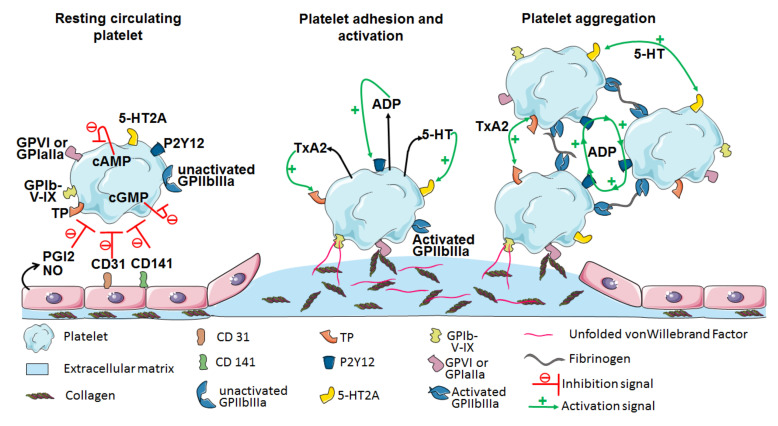
Figure 1.
Platelets’ central role in hemostasis. Under resting conditions, platelets circulate in the vessel and are kept in a quiescent state by unaltered endothelial cells that express CD31 (i.e., Platelet endothelial cell adhesion molecule-1, PECAM-1) and CD141 (i.e., thrombomodulin) and secrete prostacyclin (PGI2) and nitric oxide (NO). PGI2 and NO induce the platelet production and intracellular accumulation of cyclic AMP (cAMP) and cyclic GMP (cGMP), respectively, which are both involved in platelet inhibition. Upon vascular disruption and the exposure of the underlying extracellular matrix, platelets bind to unfolded von Willebrand factor (vWF) via their glycoprotein Ib-V-IX (GPIb-V-IX) complex. This initial adhesion is then stabilized by the direct interaction between collagen and platelet GPVI or GPIaIIa. Platelet adhesion can also be stabilized indirectly through the action of the vWF bound to collagen, which is then sensed by the GPIb-V-IX complex. Platelet adhesion then triggers multiple signaling pathways, resulting in platelet activation through the degranulation of numerous factors, including adenosine diphosphate (ADP), thromboxane A2 (TxA2) and serotonin (5-HT), through the P2Y12, TP and 5-HT2A receptors, respectively. Aside from degranulation, GPIIbIIIa undergoes a conformational change to its active form and binds to fibrinogen. The platelet secretion of hemostatic factors and their binding to fibrinogen fuel the autocrine activation loop of platelets, leading to aggregation and coagulation processes.