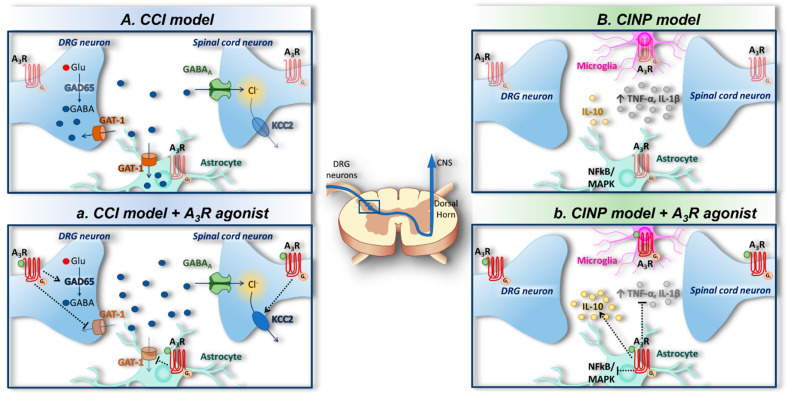
Figure 2.
Adenosine A3 receptors and spinal mechanisms of pain control. (A) Chemotherapy-induced neuropathic pain (CINP) is characterized by high neuroexcitatory/proinflammatory cytokine (TNF-α and IL-1β) release and the activation of NFκB and MAPK in the spinal dorsal horn. (a) Glial A3Rs inhibit NFκB and MAPK activation, thus leading to a decreased release of pro-inflammatory TNF-α and IL-1β and an increased release of anti-inflammatory interleukin-10 (IL-10) [52]. (B) Chronic constriction injury (CCI), an animal model of neuropathic pain, induces deregulation of γ-aminobutyric acid (GABA) signaling via a reduction in GABA synthesis by glutamic acid decarboxylase 65-kDa (GAD65), and enhanced GABA reuptake via the GABA transporter 1- (GAT-1) and the loss of Cl− gradient by K+-Cl− co-transporter 2 (KCC2) inhibition, leading to an overall reduction in GABA at the synapse. (b) A3 receptor (A3R) activation prevents CCI-induced GAT-1 enhancement and GAD65 inhibition as well as KCC2 loss of function [51], augmenting extracellular GABA levels.