Abstract
Aims
The aims of the study were to compare clinical outcomes and valve durability after 8 years of follow-up in patients with symptomatic severe aortic valve stenosis at low surgical risk treated with either transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement (SAVR).
Methods and results
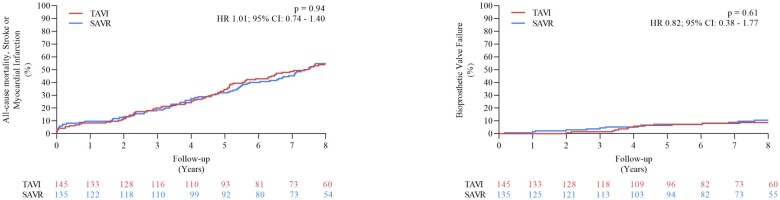
In the NOTION trial, patients with symptomatic severe aortic valve stenosis were randomized to TAVI or SAVR. Clinical status, echocardiography, structural valve deterioration, and failure were assessed using standardized definitions. In total, 280 patients were randomized to TAVI (n = 145) or SAVR (n = 135). Baseline characteristics were similar, including mean age of 79.1 ± 4.8 years and a mean STS score of 3.0 ± 1.7%. At 8-year follow-up, the estimated risk of the composite outcome of all-cause mortality, stroke, or myocardial infarction was 54.5% after TAVI and 54.8% after SAVR (P = 0.94). The estimated risks for all-cause mortality (51.8% vs. 52.6%; P = 0.90), stroke (8.3% vs. 9.1%; P = 0.90), or myocardial infarction (6.2% vs. 3.8%; P = 0.33) were similar after TAVI and SAVR. The risk of structural valve deterioration was lower after TAVI than after SAVR (13.9% vs. 28.3%; P = 0.0017), whereas the risk of bioprosthetic valve failure was similar (8.7% vs. 10.5%; P = 0.61).
Conclusions
In patients with severe aortic valve stenosis at low surgical risk randomized to TAVI or SAVR, there were no significant differences in the risk for all-cause mortality, stroke, or myocardial infarction, as well as the risk of bioprosthetic valve failure after 8 years of follow-up.
Clinical trial registration
URL: http://www.ClinicalTrials.gov. Unique identifier: NCT01057173.
Keywords: Surgical aortic valve replacement, Transcatheter aortic valve implantation, Bioprosthetic aortic valve durability, Mortality, Stroke
Graphical Abstract
Clinical and aortic bioprosthetic valve failure 8 years after transcatheter and surgical aortic valve replacement. CI, confidence interval; HR, hazard ratio.
See page 2920 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab393)
Introduction
Transcatheter aortic valve implantation (TAVI) has expanded rapidly for the treatment of symptomatic severe aortic valve stenosis (AS) after multiple randomized clinical trials have demonstrated TAVI to be either non-inferior or superior to surgical aortic valve replacement (SAVR) in short- and mid-term outcomes.1–8 Most recently, two major randomized clinical trials reported that TAVI was non-inferior to SAVR when comparing the risk for all-cause mortality or disabling stroke in patients at low surgical risk—and even superior when including valve-related re-hospitalisation.4 , 5 Consequently, TAVI was approved by the American Food and Drug Administration (FDA) in 2019 for patients with symptomatic severe AS and low surgical risk, and recently included in the American College of Cardiology (ACC)/American Heart Association (AHA) guideline for the management of patients with valvular heart disease.9 Thus, TAVI is now endorsed in the United States as a viable treatment option for patients with symptomatic severe AS across the full range of surgical mortality risk groups.1 , 9 , 10
The limited data on long-term clinical outcome after TAVI compared to SAVR, as well as the durability of transcatheter heart valves (THV), have been raised as a concern for expansion of TAVI to patients with longer life expectancy.11 , 12 Despite this, the use of TAVI in younger patients at low surgical risk is unlikely to be delayed, and therefore long-term data are increasingly important.
The Nordic Aortic Valve Intervention (NOTION) trial randomized patients at lower surgical risk to TAVI or SAVR between 2010 and 2013. Expanding on previous mid-term follow-up data from the NOTION trial,2 , 13 the aims of the present study were to report clinical outcomes and durability of bioprosthetic aortic valves 8 years after TAVI and SAVR.
Methods
The NOTION trial is an investigator-initiated, unblinded, randomized clinical trial conducted at hospitals in Denmark and Sweden.14 All participants provided informed consent. The trial was approved by local ethics committees. All data have been monitored and an independent clinical events committee adjudicated clinical events. The trial is registered with ClinicalTrials.gov, NCT01057173.
Patients
Patients aged ≥70 years with severe AS assessed by the local heart team were included in the trial. A detailed list of inclusion and exclusion criteria have been described previously.15 Enrolled patients were randomized to SAVR using bioprosthetic valves (St Jude Medical Epic®, 29%; Medtronic Mosaic®, 27%; St Jude Medical Trifecta®, 24%; Carpentier-Edwards Perimount®, 10%; and Sorin Mitroflow®, 10%) or TAVI using the first-generation self-expanding Medtronic CoreValve® in all patients. Patients were followed with clinical and echocardiographic visits at 3 and 12 months, and then yearly after the index procedure.
Outcome definitions
Valve durability was divided into bioprosthetic valve dysfunction (BVD) and bioprosthetic valve failure (BVF) based on the consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI), the European Society of Cardiology (ESC), and the European Association for Cardio-thoracic Surgery (EACTS).16
Bioprosthetic valve dysfunction was categorized into four groups: (i) structural valve deterioration (SVD) defined as moderate SVD (mean transvalvular gradient ≥20 mmHg, increase in mean gradient ≥10 mmHg from 3 months post-procedure, or new or worsening moderate intra-prosthetic aortic regurgitation from 3 months post-procedure) and severe SVD (mean transvalvular gradient ≥40 mmHg, increase in mean gradient ≥20 mmHg from 3 months post-procedure, or new or worsening severe intra-prosthetic aortic regurgitation from 3 months post-procedure); (ii) non-structural valve deterioration (NSVD) defined as moderate to severe patient-prosthesis mismatch (PPM) (indexed effective orifice area ≤0.85 cm2/m2 for moderate PPM and ≤0.65 cm2/m2 for severe PPM) at 3 months, or more than mild paravalvular leakage (PVL); (iii) bioprosthetic valve thrombosis defined as thrombus development on any structure of the prosthetic valve leading to dysfunction; and (iv) infectious endocarditis diagnosed according to the modified Duke criteria.17
Bioprosthetic valve failure was defined as one of the following three criteria: (i) valve-related death (death caused by BVD or sudden unexplained death following diagnosis of BVD); (ii) severe hemodynamic SVD; and (iii) aortic valve re-intervention following diagnosis of BVD.
To avoid that PPM would impact the classification of SVD, a modified definition of SVD was applied using a mean gradient ≥20 mmHg and an increase in mean gradient ≥10 mmHg after 3 months post-procedure.
Statistics
Continuous variables are presented as mean ± standard deviation and compared using Student’s t-test, median with interquartile range, or Wilcoxon signed-rank test. Categorical variables were presented as counts and percentages and compared with the chi-square or Fisher’s exact test. Survival analyses were performed using the Kaplan–Meier method and compared using the log-rank test. For analyses with death a competing risk, the absolute risk was analysed using the Aalen-Johansen method and groups were compared using Gray’s test. Cox regression was used to analyse the association of exposure with mortality rates and reported as hazard ratio (HR) with 95% confidence interval (CI). The clinical outcome of all-cause mortality, stroke or myocardial infarction was reported based on the intention-to-treat population. All available data on valve durability were reported based on the as-implanted population (Supplementary material online, Figure S1). Data were censored after 8 years of follow-up.
All statistical analyses were performed with SAS Enterprise Guide 7.15 (SAS Institute, Cary, NC, USA) and the null hypothesis was rejected on P-values <0.05.
Results
A total of 280 patients were randomized 1:1 to TAVI (n = 145) or SAVR (n = 135) in the intention-to-treat population. Four patients died before the planned intervention (three patients allocated to TAVI and one patient allocated to SAVR). Three patients randomized to TAVI crossed over to SAVR peri-procedurally due to complications and two SAVR patients ended up not having a bioprosthetic valve implanted, resulting in 274 patients in the as-implanted population (139 TAVI and 135 SAVR) (Supplementary material online, Figure S1).
The baseline characteristics of the intention-to-treat population have been described previously15 (Supplementary material online, Table S1); and the baseline characteristics of the as-treated population are shown in Supplementary material online, Table S2. There were no significant baseline differences between patients in the TAVI and SAVR groups. The mean age was 79.1 ± 4.8 years and Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 3.0 ± 1.7%.
The follow-up compliance for the total trial population is 98.9%, as two patients in the TAVI group were lost to follow-up after 4.8 and 5.0 years and one patient in the SAVR group was lost to follow-up after 5.4 years. Out of 133 patients still alive after 8 years, 12 patients have not yet reached the 8-year follow-up visit. Eight-year echocardiographic data were available in 102 of the 121 patients (84.3%) that had reached 8 years of follow-up, with missing data of mean gradient in eight patients (four TAVI patients and four SAVR patients), and missing data for intra-prosthetic aortic regurgitation in one TAVI patient and three SAVR patients.
Clinical outcomes
After 8 years of follow-up, there was no difference between the estimated risk of all-cause mortality for patients in the TAVI and SAVR groups (51.8% vs. 52.6%, P = 0.90; HR 0.98, 95% CI 0.71–1.36) (Figure 1 and Table 1) or the cumulative incidence of cardiovascular death (40.6% vs. 43.6%, P = 0.64; HR 0.93, 95% CI 0.64–1.34). Both the cumulative incidence of stroke (8.3% vs. 9.1%, P = 0.90; HR 0.93, 95% CI 0.42–2.08) and myocardial infarction (6.2% vs. 3.8%, P = 0.33; HR 1.70, 95% CI 0.57–5.07) were also similar after TAVI and SAVR. The estimated risk of the composite outcome of all-cause mortality, stroke or myocardial infarction was 54.5% after TAVI and 54.8% after SAVR (P = 0.94) (HR 1.01, 95% CI 0.74–1.40) (Figure 2 and Graphical abstract). Complication rates after TAVI and SAVR are shown in Table 1. The risk of all-cause mortality for patients with a permanent pacemaker implantation within 30 days and patients without permanent pacemaker after TAVI was 54.4% vs. 45.5% (P = 0.20) (HR 1.39, 95% CI 0.84–2.30). Based on the degree of PVL at 3 months after TAVI, the risk of all-cause mortality at 8 years was similar in patients with moderate-to-severe PVL (20 patients, 13.8%) compared to patients with no/trace/mild PVL (111 patients, 76.5%) (all-cause mortality: 55.0% vs. 48.3%, P = 0.53), as well as for mild PVL (77 patients, 53.1%) compared to no/trace PVL (34 patients, 23.5%) (all-cause mortality: 48.6% vs. 48.0%, P = 0.67). There was also no significant difference in the functional class between patients undergoing TAVI or SAVR (Supplementary material online, Figure S2).
Figure 1.
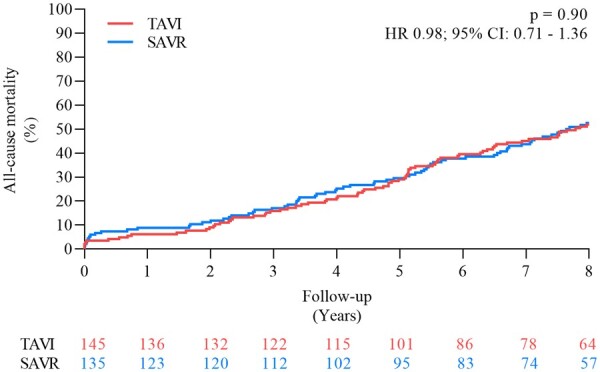
Estimated risk of all-cause mortality. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Table 1.
Complications
| TAVI | SAVR | P-value | |
|---|---|---|---|
| (n = 145) | (n = 135) | ||
| All-cause mortality | 51.8 (8.5) | 52.6 (8.7) | 0.90 |
| Cardiovascular death | 40.6 (6.6) | 43.6 (7.2) | 0.64 |
| Stroke | 8.3 (1.4) | 9.1 (1.7) | 0.90 |
| Transient ischaemic attack | 7.6 (1.3) | 5.3 (0.9) | 0.41 |
| Myocardial Infarction | 6.2 (1.1) | 3.8 (0.6) | 0.33 |
| New-onset atrial fibrillation | 50.0 (18.5) | 74.1 (53.1) | <0.0001 |
| New permanent pacemaker | 42.5 (11.0) | 10.9 (1.9) | <0.0001 |
Risk estimates are % and (per 100 person-years).
SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation
Figure 2.
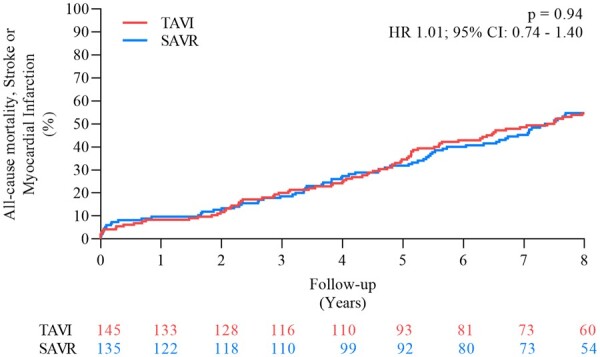
Estimated risk of all-cause mortality, stroke or myocardial infarction. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Echocardiographic outcomes and valve durability
In the as-implanted population, patients with an implanted THV had a larger effective orifice area and a lower mean gradient at every yearly follow-up up to 8 years as compared to patients treated with SAVR (Figure 3). The mean left ventricular ejection fraction was 52 ± 8.0% in TAVI patients and 54 ± 8.2% in SAVR patients after 8 years of follow-up (P = 0.14).
Figure 3.
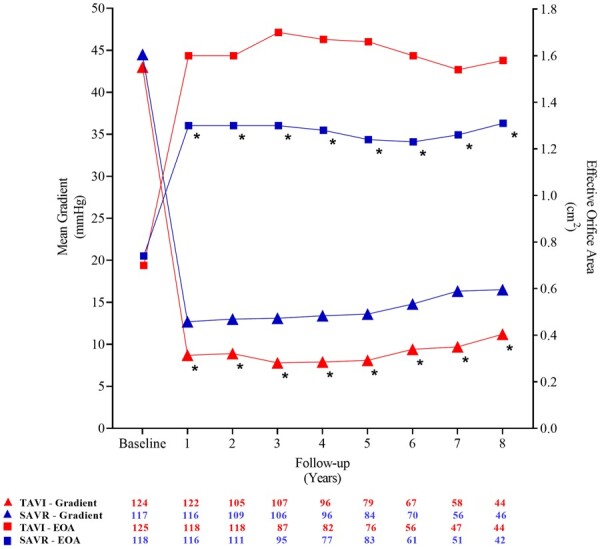
Mean gradient and effective orifice area during follow-up. EOA, effective orifice area; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation. *P < 0.05.
The cumulative incidence of SVD was 13.9% after TAVI and 28.3% after SAVR (P = 0.0017) (HR 0.42, 95% CI 0.24–0.72) (Figure 4). The components of SVD are shown in Table 2. By applying the modified definition of SVD, the cumulative incidence of SVD was 8.8% after TAVI and 15.7% after SAVR (P = 0.068) (HR 0.51, 95% CI 0.25–1.04).
Figure 4.
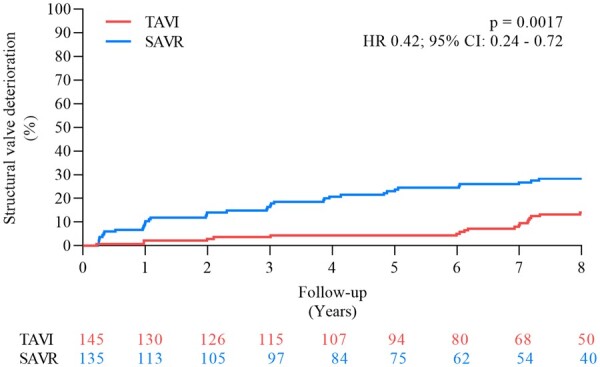
Structural valve deterioration. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Table 2.
Structural valve deterioration
| TAVI | SAVR | P-value | |
|---|---|---|---|
| (n = 139) | (n = 135) | ||
| Structural valve deterioration | 13.9 | 28.3 | 0.0017 |
| Moderate structural valve deterioration | 13.2 | 27.5 | 0.0016 |
| − Mean gradient ≥20 mmHg | 8.7 | 26.8 | <0.0001 |
| − Mean gradient ≥10 and <20 mmHg change from 3 months | 6.6 | 14.2 | 0.035 |
| − Moderate intraprosthetic AR | 3.7 | 0 | 0.028 |
| Severe structural valve deterioration | 2.2 | 6.8 | 0.068 |
| − Mean gradient ≥20 mmHg | 0.7 | 3.8 | 0.091 |
| − Mean gradient ≥20 mmHg change from 3 months | 1.5 | 6.8 | 0.027 |
| − Severe intraprosthetic AR | 0 | 0 | – |
Risk estimates are %.
AR, aortic valve regurgitation; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
BVD developed in 62.0% of patients undergoing TAVI as compared to 70.5% in patients receiving a surgical bioprosthesis (P = 0.064) (Table 3). NSVD was a major component of BVD and was due to moderate to severe PPM (moderate-to-severe PPM: 43.9% and 60.7%, P = 0.0049; moderate PPM: 30.9% and 32.6%, P = 0.72; severe PPM: 13.0% and 28.2%, P = 0.0021), and more than mild PVL (moderate-to-severe PVL: 21.6% and 1.5%, P < 0.0001; moderate PVL: 21.6% and 1.5%, P < 0.0001; severe PVL: 0.7% and 0.0%, P = 0.32) for patients treated with TAVI and SAVR, respectively.
Table 3.
Bioprosthetic valve dysfunction
| TAVI | SAVR | P-value | |
|---|---|---|---|
| (n = 139) | (n = 135) | ||
| Bioprosthetic valve dysfunction | 62.0 | 70.5 | 0.064 |
| − Structural valve deterioration | 13.9 | 28.6 | 0.0017 |
| − Non-structural valve deterioration | 54.7 | 60.7 | 0.18 |
| − Thrombosis | 0 | 0 | – |
| − Endocarditis | 7.2 | 7.4 | 0.95 |
Risk estimates are %.
SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
No patient developed clinical valve thrombosis, whereas the cumulative incidence of endocarditis was 7.2% and 7.4% (P = 0.95) for patients treated with TAVI and SAVR, respectively.
The cumulative incidence of BVF was 8.7% for patients that had a THV implanted and 10.5% for patients with a surgical bioprosthesis (P = 0.61) (HR 0.82; 95% CI 0.38–1.77) (Figure 5 and Graphical abstract). The components of BVF through 8 years of follow-up are shown in Table 4.
Figure 5.
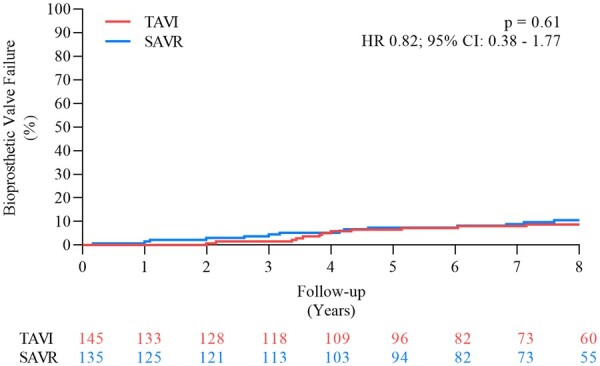
Bioprosthetic valve failure. CI, confidence interval; HR, hazard ratio; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation.
Table 4.
Bioprosthetic valve failure
| TAVI | SAVR | P-value | |
|---|---|---|---|
| (n = 139) | (n = 135) | ||
| Bioprosthetic valve failure | 8.7 | 10.5 | 0.61 |
| − Valve-related death | 5.0 | 3.7 | 0.60 |
| − Severe structural valve deterioration | 2.2 | 6.8 | 0.068 |
| − Aortic valve re-intervention | 3.6 | 2.3 | 0.51 |
Risk estimates are %.
SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation
Discussion
To date, available long-term data from other randomized clinical trials comparing TAVI vs. SAVR are limited to 5 years of follow-up with an all-cause mortality rate of 46.0–67.8% after TAVI and 42.1-62.4% after SAVR.6–8 The NOTION trial randomized patients with severe AS and without a prespecified surgical risk profile, resulting in >80% of the patients with a STS-PROM score below 4% regarded as low surgical risk. The rate of all-cause mortality after 5 years of follow-up was 27.6% after TAVI and 28.9% after SAVR, allowing for longer follow-up time and comparison.
The long-term clinical outcome of the NOTION trial demonstrated that there were no significant differences in the risk of all-cause mortality, stroke, and myocardial infarction between patients with symptomatic severe AS and lower surgical risk after TAVI as compared to SAVR during 8 years of follow-up. Outcomes on bioprosthetic durability demonstrated that the risk of SVD was lower after TAVI compared to SAVR, whereas there was no significant difference in the risk of BVF between the two groups. These results are the longest reported follow-up of a patient population randomized to TAVI or SAVR.
Clinical outcomes
TAVI has been extensively investigated and was non-inferior or even superior to SAVR across all surgical risk groups when comparing the risk of short- and mid-term all-cause mortality or disabling stroke.2–8 Based on the results of two industry-driven randomized clinical trials including elderly patients only with STS-PROM score below 4%, the FDA approved TAVI in patients at low surgical risk in 2019. Recent data from the STS-ACC registry showed that the annual volume of performed TAVI procedures in patients with low surgical risk has started to grow rapidly similar to the volume observed for patients with intermediate surgical risk following the FDA approval of TAVI in this population in 2017.18
The long-term outcome after TAVI compared to SAVR is still largely unknown due to the high burden of comorbidities, advanced age, and thereby a relative short life expectancy of the patients included in the early randomized clinical trials. Despite the favourable short- and mid-term outcomes after TAVI compared to SAVR, concerns have been raised on several issues related to TAVI which may have a potential detrimental impact on long-term outcomes. The risk of conduction abnormalities, including left bundle branch block or the need for a permanent pacemaker, remains higher after TAVI than after SAVR.4 , 5 , 18 Conduction abnormalities have been found to increase the risk of all-cause mortality and heart failure re-hospitalizations when compared to TAVI patients without conduction abnormalities.19 , 20 In the present study, left ventricular ejection fraction was similar between TAVI and SAVR patients after 8 years of follow-up and the risk of all-cause mortality was not significant, although numerically higher in pacemaker-naive patients that underwent permanent pacemaker implantation within 30 days after TAVI compared to patients without a permanent pacemaker. A lower threshold for prophylactic pacemaker implantation in the early TAVI period could have caused a low pacing percentage potentially ameliorating the impact of pacemaker implantation. However, pacing percentages were not available in the present study to confirm this. The rate of more than mild PVL after TAVI was higher in the NOTION trial than in contemporary practice, which may partly be explained by aortic annulus sizing performed by echocardiography and not computed tomography, and the use of primarily first-generation THV without outer sealing skirt and the possibility of re-positioning. The presence of PVL was not associated with an increased risk of death after 8 years of follow-up. Still, the risk of all-cause mortality was increased for TAVI patients with mild vs. no or trace PVL in the 5-year data from the PARTNER 1 trial (73.0% vs. 68.3%; P = 0.003) and PARNTER 2 trial (48.7% vs. 41.1%; P = 0.07).6 , 8 This trend towards a higher mortality in patients with PVL after TAVI may be worrisome for younger patients with longer life expectancy.
Bioprosthetic durability
As TAVI is now indicated for patients with a longer life expectancy, these patients are more likely to outlive the implanted bioprosthetic valve. The long-term clinical consequence of re-intervention is still unknown and further long-term data are needed. The ACC/AHA guideline for the management of patients with valvular heart disease has recently been updated and now recommends a lower age limit of 65 years for TAVI due to the lack of long-term data on THV durability.9 Using the same definition of SVD and BVF as in the current study, other registries have reported the risk of severe SVD after TAVI to be 2.4–4.5% and the risk of BVF to be 0.6–4.5% during 8 years of follow-up.21 The durability of surgical bioprosthetic aortic valves is generally thought to be at least 10 years. However, BVF for surgical bioprostheses has previously been defined as re-intervention, with a risk of under-estimating the incidence of SVD as some patients might not be offered re-valving due to high age, comorbidity burden, or frailty.9 , 22 Therefore, randomized data using standardized definition of bioprosthetic valve deterioration is paramount in order to evaluate durability. Current data on the durability of THV compared to surgical bioprosthesis from randomized trials are limited to 5–6 years.7 , 13 , 23 In the present study with 8 years of follow-up, the risk of SVD was lower after TAVI than after SAVR. A similar incidence and significant lower risk of SVD 5 years after TAVI compared to SAVR was found in the CoreValve U.S. Pivotal High Risk trial, which used the same definition of SVD.7 However, the risk of BVF remains similar between TAVI and SAVR patients in both trials. This might be due to a higher risk of PPM after SAVR increasing the immediate post-procedure mean gradient above the threshold for moderate SVD, without the presence of actual structural changes. A recent study reported that the second-generation balloon expandable SAPIEN XT THV had a higher risk of SVD whereas the third-generation SAPIEN 3 THV had similar rate of SVD compared to surgical bioprostheses. The study used a recently published standardized definition of SVD from the Valve Academic Research Consortium 3 that emphasized on defining the cause for SVD.23 , 24 In the alternative definition of SVD and BVF, there must be a permanent morphological change of the bioprosthesis (e.g. leaflet tear, disruption, flail leaflet, leaflet fibrosis or calcification) in addition to an increase in mean gradient ≥10 mmHg from 1 to 3 months post-procedure resulting in a mean gradient ≥20 mmHg with a concomitant reduction in aortic valve area ≥0.3 cm2. Including early post-procedure echo data could help differentiate between NSVD such as PPM and early SVD. In the present study, using a modified definition of SVD to account for the number of patients with PPM, the risk for SVD was still clinically lower but not significantly different for TAVI compared to SAVR.
The risk of endocarditis remains low and similar between TAVI and SAVR patients after 8 years of follow-up. In a large Danish national registry including 2632 TAVI and 3777 matched SAVR patients, the risk of endocarditis was 5.8% and 5.1% after 5 years of follow-up, respectively,25 comparable to the 5-year incidence of endocarditis of 5.8% for TAVI patients and 5.9% for SAVR patients in the NOTION trial.13
The risk of BVF, including the risk of aortic valve re-intervention but also the development of severe hemodynamic SVD assessed during yearly echocardiography, was low and similar for patients undergoing TAVI or SAVR after 8 years of follow-up. Although even longer-term data are needed, the findings from the present study are reassuring for the expansion of TAVI to patients with longer life expectancy.
Study limitations
Many patients screened for the NOTION trial were excluded, most frequently due to significant coronary artery disease, prohibitive high surgical risk, or technical unsuitability for intervention at the time of the index procedures. Trial eligibility and THV sizing were based on transthoracic echocardiography instead of computed tomography imaging. Echocardiographic data were not adjudicated by a core laboratory. Only the first-generation CoreValve self-expanding prosthesis was used for TAVI which could have impacted the outcome after TAVI when compared to newer generations of THVs with improved design features including sealing skirts, reduced profile of delivery catheters, and repositionability of the THV.3–5 Furthermore, several different bioprosthesis were chosen for SAVR at the surgeon’s discretion, including the Mitroflow and Trifecta bioprostheses, which have been reported to have a higher risk of earlier SVD.26 , 27 This could have negatively affected the rate of SVD for SAVR and limited the extrapolation of clinical and echocardiographic outcomes to other bioprosthetic aortic valves. Surgical annular enlargement techniques were not applied in the trial, which may have led to the high rate of PPM in the SAVR group. The trial was designed with a primary outcome after 1 year, and therefore, the current study is an exploratory analysis in a limited population of 121 patients alive at 8 years.
Conclusion
The NOTION trial included patients with symptomatic severe AS at low surgical risk. After 8 years of follow-up, the risk of all-cause mortality, stroke or myocardial infarction as a composite outcome, and the individual components were not significantly different after TAVI and SAVR. The risk of SVD was significantly lower after TAVI than after SAVR, and the rate of BVF continued to be low and not different in both groups.
The long-term results are reassuring for TAVI both regarding clinical outcomes and THV durability, as TAVI is now indicated for patients with longer life expectancy. Still, further long-term data are needed including data on all types of THVs.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the Danish Heart Foundation [grant numbers: 09-10-AR76-A2733-25400, 12-04-R90-A3879-22733, and 13-04-R94-A4473-22762] and Medtronic.
Conflict of interest: T.H.J. has received a research grant from Edwards Lifesciences. L.S. reports grants and personal fees from Medtronic, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Data availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Supplementary Material
Contributor Information
Troels Højsgaard Jørgensen, Department of Cardiology, The Heart Centre, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, Copenhagen 2100, Denmark.
Hans Gustav Hørsted Thyregod, Department of Cardiothoracic Surgery, The Heart Centre, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, Copenhagen 2100, Denmark.
Nikolaj Ihlemann, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Denmark.
Henrik Nissen, Department of Cardiology, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Denmark.
Petur Petursson, Department of Cardiology, Sahlgrenska University Hospital, Blå stråket 5, 413 45 Gothenburg, Sweden.
Bo Juel Kjeldsen, Department of Cardiothoracic and Vascular Surgery, Odense University Hospital, J. B. Winsløws Vej 4, 5000 Odense, Denmark.
Daniel Andreas Steinbrüchel, Department of Medicine, Nykoebing F Hospital and University of Southern Denmark, J. B. Winsløws Vej 4, 5000 Odense, Denmark.
Peter Skov Olsen, Department of Cardiothoracic Surgery, The Heart Centre, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, Copenhagen 2100, Denmark.
Lars Søndergaard, Department of Cardiology, The Heart Centre, Rigshospitalet, Copenhagen University Hospital, Blegdamsvej 9, Copenhagen 2100, Denmark.
References
- 1. Jørgensen TH. Transcatheter aortic valve replacement in patients with lower surgical risk. JACC Cardiovasc Interv 2020;13:332–334. [DOI] [PubMed] [Google Scholar]
- 2. Thyregod HGH, Ihlemann N, Jørgensen TH, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Steinbruüchel DA, Olsen PS, Søndergaard L. Five-year clinical and echocardiographic outcomes from the NOTION randomized clinical trial in patients at lower surgical risk. Circulation 2019;139:2714–2723. [DOI] [PubMed] [Google Scholar]
- 3. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PWJC, Kappetein AP; SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 4. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 5. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 6. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Davidson MJ, Svensson LG, Akin J; PARTNER 1 Trial Investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477–2484. [DOI] [PubMed] [Google Scholar]
- 7. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, Kleiman NS, Chetcuti S, Hermiller JB, Heiser J, Merhi W, Zorn GL, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte JV, Mumtaz M, Oh JK, Huang J, Adams DH; CoreValve U.S. Pivotal High Risk Trial Clinical Investigators. 5-Year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol 2018;72:2687–2696. [DOI] [PubMed] [Google Scholar]
- 8. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, Yoon S-H, Trento A, Svensson LG, Herrmann HC, Szeto WY, Miller DC, Satler L, Cohen DJ, Dewey TM, Babaliaros V, Williams MR, Kereiakes DJ, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Brown DL, Fearon WF, Russo MJ, Pibarot P, Hahn RT, Jaber WA, Rogers E, Xu K, Wheeler J, Alu MC, Smith CR, Leon MB; PARTNER 2 Investigators. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med 2020;382:799–809. [DOI] [PubMed] [Google Scholar]
- 9. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35–e71. [DOI] [PubMed] [Google Scholar]
- 10. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 11. Otto CM. Informed shared decisions for patients with aortic stenosis. N Engl J Med 2019;380:1769–1770. [DOI] [PubMed] [Google Scholar]
- 12. Sondergaard L. Transcatheter aortic valve implantation in patients with longer life expectancy: what measures are needed? Eur Heart J 2019;40:1331–1333. [DOI] [PubMed] [Google Scholar]
- 13. Søndergaard L, Ihlemann N, Capodanno D, Jørgensen TH, Nissen H, Kjeldsen BJ, Chang Y, Steinbrüchel DA, Olsen PS, Petronio AS, Thyregod HGH. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. J Am Coll Cardiol 2019;73:546–553. [DOI] [PubMed] [Google Scholar]
- 14. Thyregod HG, Søndergaard L, Ihlemann N, Franzen O, Andersen LW, Hansen PB, Olsen PS, Nissen H, Winkel P, Gluud C, Steinbrüchel DA. The Nordic Aortic Valve Intervention (NOTION) trial comparing transcatheter versus surgical valve implantation: study protocol for a randomised controlled trial. Trials 2013;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thyregod HGH, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Søndergaard L. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis. J Am Coll Cardiol 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 16. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, Lancellotti P, Sondergaard L, Ludman PF, Tamburino C, Piazza N, Hancock J, Mehilli J, Byrne RA, Baumbach A, Kappetein AP, Windecker S, Bax J, Haude M. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;38:3382–3390. [DOI] [PubMed] [Google Scholar]
- 17. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994;96:200–209. [DOI] [PubMed] [Google Scholar]
- 18. Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, Deeb GM, Thourani VH, Cohen DJ, Desai N, Kirtane AJ, Fitzgerald S, Michaels J, Krohn C, Masoudi FA, Brindis RG, Bavaria JE. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol 2020;76:2492–2516. [DOI] [PubMed] [Google Scholar]
- 19. Jørgensen TH, De Backer O, Gerds TA, Bieliauskas G, Svendsen JH, Søndergaard L. Mortality and heart failure hospitalization in patients with conduction abnormalities after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019;12:52–61. [DOI] [PubMed] [Google Scholar]
- 20. Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard L, Jørgensen TH, Lopez-Aguilera J, Kodali S, Leon M, Nazif T, Rodés-Cabau J. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J 2020;41:2771–2781. [DOI] [PubMed] [Google Scholar]
- 21. Sawaya F, Jørgensen TH, Søndergaard L, De Backer O. Transcatheter bioprosthetic aortic valve dysfunction: what we know so far. Front Cardiovasc Med 2019;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez-Gabella T, Voisine P, Puri R, Pibarot P, Rodés-Cabau J. Aortic bioprosthetic valve durability. J Am Coll Cardiol 2017;70:1013–1028. [DOI] [PubMed] [Google Scholar]
- 23. Pibarot P, Ternacle J, Jaber WA, Salaun E, Dahou A, Asch FM, Weissman NJ, Rodriguez L, Xu K, Annabi M-S, Guzzetti E, Beaudoin J, Bernier M, Leipsic J, Blanke P, Clavel M-A, Rogers E, Alu MC, Douglas PS, Makkar R, Miller DC, Kapadia SR, Mack MJ, Webb JG, Kodali SK, Smith CR, Herrmann HC, Thourani VH, Leon MB, Hahn RT; PARTNER 2 Investigators. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol 2020;76:1830–1843. [DOI] [PubMed] [Google Scholar]
- 24. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Mieghem NMV, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J 2021. [DOI] [PubMed] [Google Scholar]
- 25. Butt JH, Ihlemann N, Backer OD, Søndergaard L, Havers-Borgersen E, Gislason GH, Torp-Pedersen C, Køber L, Fosbøl EL. Long-term risk of infective endocarditis after transcatheter aortic valve replacement. J Am Coll Cardiol 2019;73:1646–1655. [DOI] [PubMed] [Google Scholar]
- 26. Minami K, Zittermann A, Schulte-Eistrup S, Koertke H, Körfer R. Mitroflow synergy prostheses for aortic valve replacement: 19 years experience with 1,516 patients. Ann Thorac Surg 2005;80:1699–1705. [DOI] [PubMed] [Google Scholar]
- 27. Kaneyuki D, Nakajima H, Asakura T, Yoshitake A, Tokunaga C, Tochii M, Hayashi J, Takazawa A, Izumida H, Iguchi A. Early first-generation trifecta valve failure: a case series and a review of the literature. Ann Thorac Surg 2020;109:86–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.