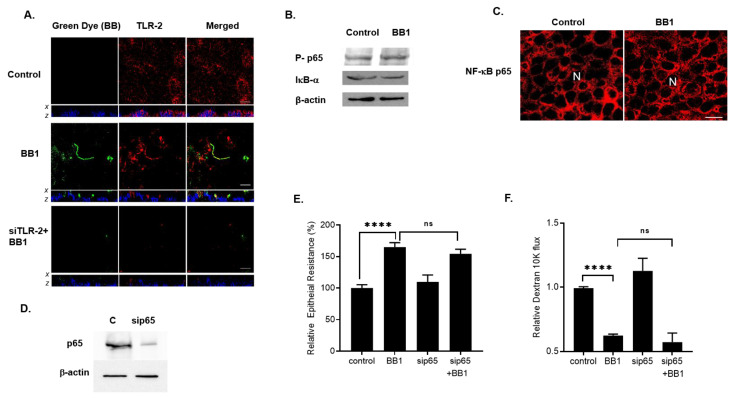
Figure 3.
Intracellular mechanisms involved in BB1 enhancement of Caco-2 intestinal epithelial TJ barrier. (A) BB1 attachment to the Caco-2 apical membrane surface. In control Caco-2 monolayers, TLR-2 (red) is diffusely distributed on the apical membrane surface. LA1 (green) adheres to Caco-2 apical membrane at sites of TLR-2 (yellow in merge panel, middle row, 2-h treatment). Nuclei are shown in blue color. x–z stacks showed that the BB1 and TLR-2 co-localized on the apical membrane of Caco-2 cells. The siRNA knock-down of TLR-2 (bottom row) showed loss of TLR-2 staining and prevention of BB1 attachment. White bars = 10 μm. (B) BB1 did not affect the activation of NF-κB p65 as assessed by phosph-p65 expression or the degradation of IκB-α in Caco-2 monolayers (1-day experimental period). (C) BB1 did not affect the cytoplasmic-to-nuclear translocation of NF-κB p65 as determined by immunofluorescence imaging, 40×. (D) NF-κB p65 siRNA transfection caused a near-complete depletion of NF-κB p65 protein expression. (E) NF-κB p65 siRNA transfection did not prevent the BB1-induced increase in Caco-2 TER and (F) decrease to dextran 10 kDa flux (1-day experimental period); **** p < 0.001 vs. control; ns: not significant.