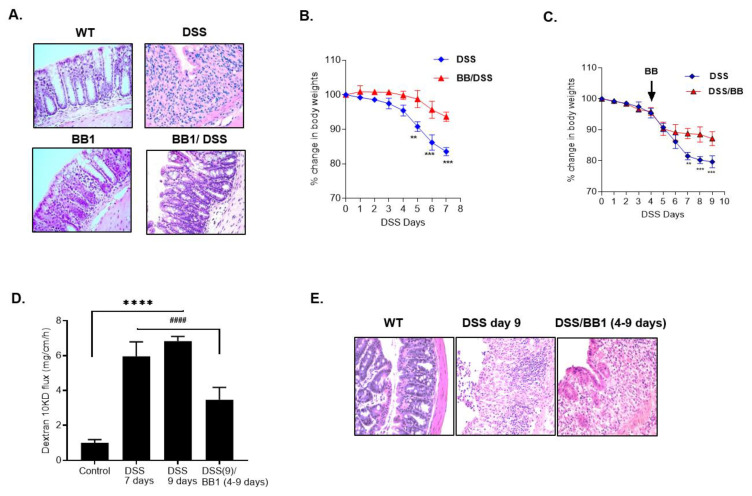
Figure 6.
Protective and therapeutic effects of BB1 on DSS-induced colitis. (A) H & E staining of colonic mucosa after DSS administration showed ulceration of colonic mucosal surface with loss of surface epithelium and crypts and diffuse infiltration of inflammatory cells. BB1 administration attenuated the DSS-induced ulceration and loss of colonic mucosal architecture and the inflammation was reduced (BB1 treatment started 2 days prior to the 7-day DSS treatment). H & E, 200×. (B) BB1 prevented the DSS-induced body weight loss. ** p < 0.01 vs. DSS; *** p < 0.001 vs. DSS. (C) BB1 treatment attenuated the DSS-induced body weight loss over the 9-day experimental period. (BB1 treatment started 4 days after DSS administration and continued up to the 9-day experimental period). ** p < 0.01 vs. DSS; *** p < 0.001 vs. DSS. (D) DSS caused a marked increase in mouse colonic permeability to dextran-10 kDa over 9 days of DSS administration, and BB1 promoted the healing from the DSS-induced increase in mouse colonic permeability over the 9-day experimental period. (BB1 administration started 4 days after DSS treatment and continued up to the 9-day experimental period) **** p < 0.0001 vs. control; #### p < 0.0001 vs. DSS. (E) H & E staining of colonic mucosa after DSS administration (9-day experimental period) showed ulceration of colonic mucosal surface with loss of surface epithelium and crypts and diffuse infiltration of inflammatory cells. BB1 treatment 4 days after DSS administration attenuated the DSS-induced inflammation (BB1 treatment started 4 days after DSS treatment and continued up to 9 days). H & E, 200×.