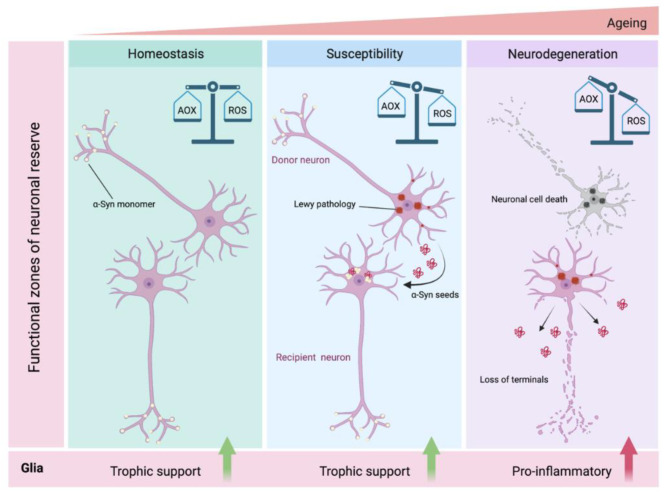
Figure 2.
Schematic depiction of hypothesized α-syn neuron-to-neuron transmission and intracellular redox imbalance resulting in neurodegeneration. Under normal homeostatic conditions, neuronal α-syn exists in soluble non-aggregated conformations and the anti-oxidant (AOX) scavenging mechanisms are at equilibrium with intracellular reactive oxygen species (ROS) generation. Misfolded α-syn perturbs cellular redox balance in favor of excessive ROS, which is further aggravated by additional susceptibility/risk factors (e.g., genetic risk factors and ageing) that promote pathological α-syn aggregation and proteopathic stress [1,2]. Subsequently, cell-to-cell transmission of α-syn seeds from the affected neurons (depicted as donor neuron) via the neuroanatomical projections onto additional neuronal populations results in transmission of α-syn pathology into the recipient neurons. In the receiving neuron, the newly internalized seeds recruit endogenous soluble α-syn and further template a vicious cycle of α-syn aggregation and neurotoxicity. In established (i.e., long-term) α-syn neuronal pathology, there is profound dysregulation of AOX/ROS balance which is associated with loss of synaptic terminals and neuronal demise. The neuroglial cells modulate these processes by providing trophic support (e.g., glia derived neurotrophic factor- GDNF) which serves to maintain pro-survival local microenvironment [185,186,187,188,189]. However, relentless disease progression and ensuing neurodegeneration are strong triggers for neuroinflammatory response. Structures are not drawn to scale. The illustration was created in biorender.com (accessed on 3 August 2021).