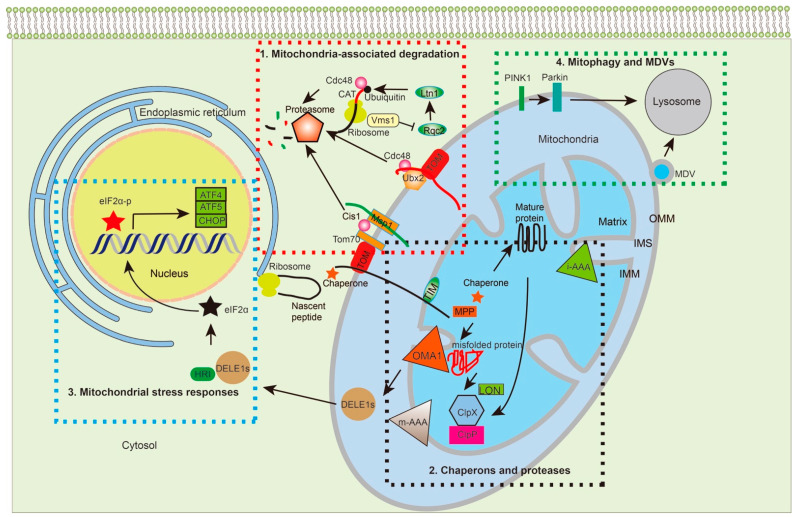
Figure 2.
Multiple MPQC pathways surveil protein synthesis, import, folding, assembly, and degradation. The MPQC surveillance system can be divided into four pathways based on studies from yeast and humans: 1. Translocation-associated protein degradation. Under standard culture conditions of yeast, Ubx2 recruits Cdc48 to the TOM complex to target prematurely folded, misfolded, or clogged proteins for proteasome-mediated degradation. Under stress conditions, the cytosolic protein Cis1 links Msp1 to Tom70, enabling Msp1 to extract the target proteins from the TOM complex for degradation. When proteins are stalled at ribosomes, Rqc2 adds CAT-tails to the stalled proteins and facilitates their ubiquitylation by Ltn1. Vms1 removes stalled proteins for degradation through preventing Rqc2 to add CAT-tails. 2. Chaperones and proteases: Chaperones and proteases are important for protein transport, folding, and degradation. Chaperones, such as Hsp70, bind to the newly synthesized proteins and prevent their folding before transporting into mitochondria. Upon transportation into the matrix, MTS is cleaved off by MPP, with mtHsp70 working with Hsp60 to further fold the protein into its functional state. m-AAA and i-AAA are located on the OMM to remove damaged proteins under stress. In the matrix, the proteases, Lon and Plpxp, regulate protein turnover and remove damaged proteins that are accumulated under stress. 3. Mitochondrial stress responses: In humans, the i-AAA protein OMA1 cleaves DELE1 into multiple DELE1s, which interact with HRI to phosphorylate eIF2α. The eIF2α-p further regulates gene expression, such as ATF4, ATF5, and CHOP, the upregulation of which further enables the coping of the stress response. 4. Mitophagy and MDVs: When mitochondrial dysfunction persists and proteostasis cannot be restored, the accumulation of dysfunctional polypeptides together with misfolded and unfolded proteins imposes a severe burden onto mitochondria. Under such conditions, PINK1 phosphorylates Parkin, which ubiquitinates the OMM for mitophagy. MDVs serve as another strategy to remove aberrant mitochondrial proteins without losing the whole organelle. TOM: translocase of the outer membrane; TIM: translocase of the inner membrane; UPS: ubiquitin-proteasome degradation system; MDVs: mitochondria-derived vesicles; PINK1: phosphatase and tensin homolog (PTEN)-induced kinase; Parkin: an E3 ubiquitin-protein ligase encoded by PARK2; i-AAA: inner membrane-embedded AAA protease; m-AAA: matrix-embedded AAA protease; MPP: matrix processing peptidase; OMA1: overlapping activity with m-AAA protease; Lon: Lon protease; Clpx: ATP-dependent Clp protease proteolytic subunit X; Clpp: ATP-dependent Clp protease proteolytic subunit P; Ubx2: UBX domain-containing protein 2; Rqc2: ribosome quality control complex subunit 2; Ltn1: E3 Ubiquitin Ligase Listerin; Cdc48: Cell division control protein 48; Cis1: citrinin sensitive knockout protein 1; ATF4: Activating Transcription Factor 4; ATF5: Activating Transcription Factor 5; CHOP: C/EBP homologous protein.