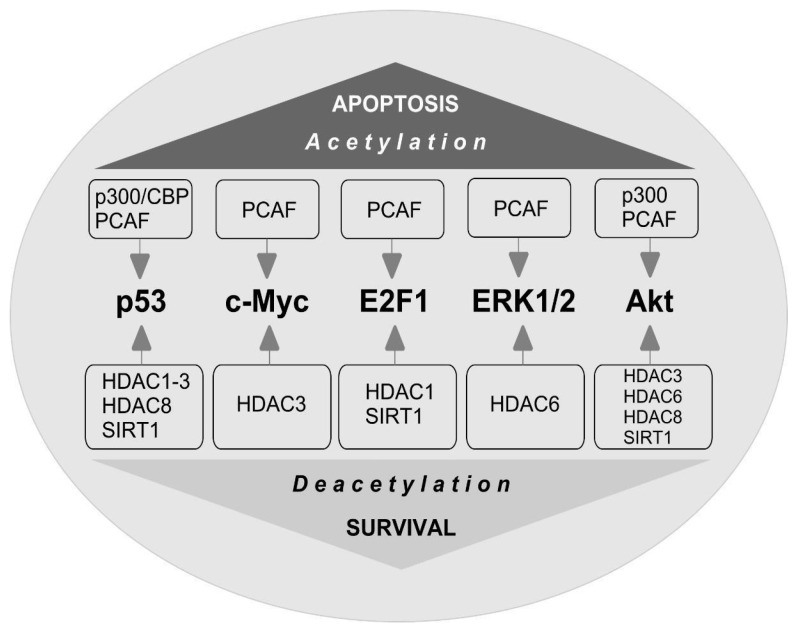
Figure 3.
Proteins coordinating the cell fate decision under acetylation/deacetylation conditions. The main proteins that play a central role in coordinating cell fate decisions are shown. p53 is acetylated by cyclic adenosine monophosphate response element-binding (CREB) protein (p300/CBP) and p300/CBP-associated factor (PCAF) acetyltransferases. Acetylation of p53 causes the activation of proapoptotic genes. Histone deacetylases HDAC1, HDAC2, HDAC3, HDAC8, and Sirtuin 1 (SIRT1) can deacetylate p53, which leads to a decrease in protein activity and repression of transcription. c-Myc is acetylated by PCAF. HDAC3 deacetylates c-Myc. c-Myc stimulates the expression of p53 and E2F1. E2F1 is acetylated by PCAF that increases protein specific binding to DNA. E2F1 is deacetylated by SIRT1 and HDAC1. Acetylation/deacetylation of E2F1 can contribute to the resistance of different types of cells to damage. ERK1/2-extracellular signal-regulated kinases are acetylated by PCAF and deacetylated by HDAC6 which prevents cell apoptosis. Akt-protein kinase Bα is acetylated by p300 and PCAF. Akt is deacetylated by HDAC-3,-6,-8 and SIRT1.