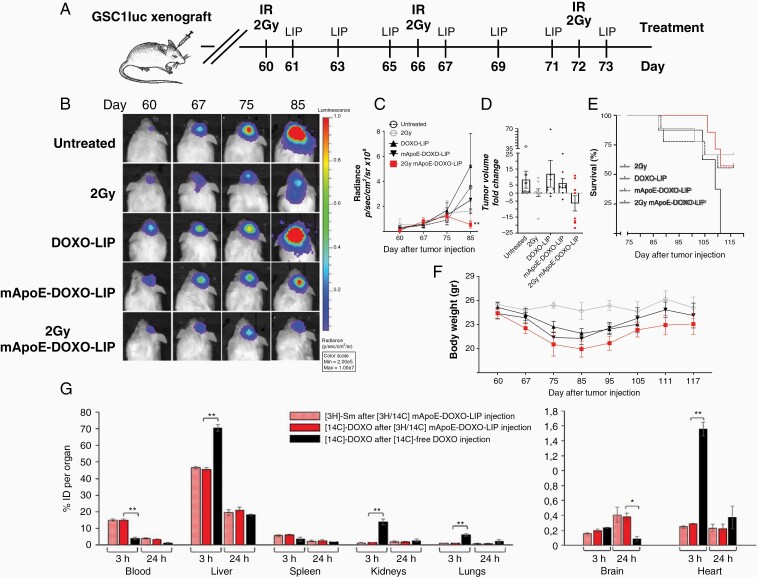
Figure 4.
In vivo efficacy of mApoE-DOXO-LIPs in the GSC-PDX model. (A) Treatments started 60 days (D60) after intracranial injection of GSC1luc cells and were administered according to the indicated schedule. GSC1luc bearing mice (n = 9 mice/group) received 35 μg/dose of encapsulated DOXO intraperitoneally. Irradiation (2Gy) was administered as whole-brain treatment. (B) Representative BLI imagines of tumor-bearing mice untreated (CTR), and treated with untargeted DOXO-LIPs (DOXO-LIP), mApoE-functionalized LIPs as single agent (mApoE-DOXO-LIP) or concomitant with radiation (2Gy/mApoE-DOXO-LIP). (C) Time course quantification of tumor BLI signals. Results are expressed as mean of radiance values ± SE detected at the indicated time points. P-values are calculated using two-way ANOVA and Tukey's multiple comparisons test (**P <.01). (D) Tumor growth/inhibition at D85 (12 days after treatment end). Data are expressed as relative BLI radiance mean values ± SE normalized to BLI values at D75. (E) Kaplan–Meier survival curve. P-log = 0.0863. (F) Body weights of the alive animals at the indicated time points. Data are presented as the mean values ± SE. (G) mApoE-DOXO-LIP distribution in peripheral organs and across intact BBB. Dually radiolabeled mApoE-DOXO-LIP ([3H]-Sm/[14C]-DOXO) or free-[14C]-DOXO were intravenously injected in healthy mice. Mice were sacrificed 3 h or 24 h after the injection. Radioactivity in peripheral organs was measured by liquid scintillation counting. The amount of [3H]-Sm or [14C]-DOXO is expressed as percentage of injected dose (ID) ± SD, *P <.05, **P < .01.