Table 3.
Parameter Estimates of the Final Model
| Pharmacokinetic Parameters | Final Model | |
|---|---|---|
| Model Fit (CV %) | Bootstrap Median (5th–95th) | |
| Absorption rate constant ka (1/h) | 15.3 (9.9) | 15.3 (13.1–17.9) |
| Clearance CL (L/h) | ||
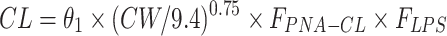 |
||
| θ1 | 0.00454 (12.2) | 0.00454 (0.00357–0.00544) |
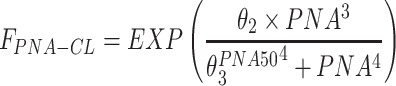 |
||
| θ2 | 106 (3.5) | 106 (101–114) |
| θ3 | 17.6 (1.2) | 17.6 (17.2–18.0) |
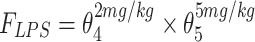 |
||
| θ4 if LPS = 2mg/kg; if LPS = 0mg/kg, θ4= 1 | 0.991 (6.0) | 0.987 (0.901–1.090) |
| θ5 if LPS = 5mg/kg; if LPS = 0mg/kg, θ5= 1 | 0.859 (6.7) | 0.859 (0.773–0.963) |
| Volume of distribution Vd (L) | ||
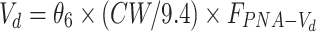 |
||
| θ6 | 0.0124 (14.9) | 0.0124 (0.0097–0.0154) |
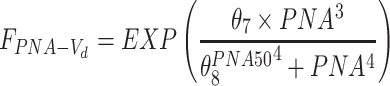 |
||
| θ7 | 53.4 (9.9) | 53.0 (45.5–62.9) |
| θ8 | 17.8 (3.0) | 17.8 (16.9–18.7) |
| Interindividual variability (%) | ||
| CL | 23.6 (7.5) | 22.9 (19.8–25.8) |
| Vd | 25.2 (13.7) | 24.3 (17.9–29.6) |
| Residual variability | ||
| Proportional (%) | 26.6 (7.9) | 26.4 (23.1–29.8) |
Abbreviations: CL, clearance; Vd, volume of distribution; CW, current body weight; PNA, postnatal age in days; FPNA-CL, FPNA-Vd, PNA-based sigmoid hyperbolic model for CL and Vd; FLPS, impact of LPS dose on CL.
