Introduction
Coronavirus disease 2019 (COVID-19)–related myositis has been described as an initial presentation and as a potential late complication of COVID-19 in adults.1 In children, rhabdomyolysis has rarely been documented during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection,2 , 3 without any detectable alteration in renal function or signs of multiorgan involvement.
We here describe the first patient with rhabdomyolysis during multisystem inflammatory syndrome temporally related to COVID-19 (MIS-C).
Patient description
This six-year-old African girl previously healthy was admitted for prominent abdominal pain with diarrhea, right chest pain, severe asthenia, and gait abnormality, diffuse and severe myalgia especially in lower limbs accentuated by muscles compression. The patient appeared exhausted and was febrile, tachypneic, and oliguric, with a distended and tender abdomen and decreased breath sounds in the right lung base.
Cardiac auscultation and blood pressure were normal. Blood tests revealed neutrophilic leukocytosis (white blood cell count: 21.38 × 109/l; neutrophils: 89%), anemia (hemoglobin: 10 g/dl), hemostatic abnormalities (platelet count: 146 × 103/μl; prothrombin time: 1.33 msec; partial thromboplastin time: 1.48 msec; D-dimer: 4.55 mg/l; plasma fibrinogen 470 mg/dl), increased markers of inflammation (C-reactive protein [CRP]: 24.86 mg/dl; procalcitonin: 57.4 ng/ml; ferritin: 506 ng/ml), high creatinine kinase levels (CK: 3392 U/l), mild acute renal failure (serum creatinine 1.11 mg/dl), signs of capillary leak syndrome (albumin: 23.7 g/dl), and hyponatremia (sodium: 131 mmol/L).
Peripheral blood smear showed rare schistocytes. Urine tests were normal, and urine myoglobin was not detected. Blood cultures were negative, as well as molecular assays for influenza, enterovirus, Epstein-Barr virus, and herpes simplex in respiratory specimens.
Chest radiograph revealed pneumonia in the right lower lobe with moderate pleural effusion. Abdominal ultrasound showed thickening of the bowel wall and mild free abdominal effusion. Echocardiography was normal.
Hemolytic uremic syndrome was suspected, so hyperhydration and antibiotic treatment were started. Reverse transcriptase polymerase chain reaction nasopharyngeal swab resulted positive for SARS-CoV-2 infection.
Based on elevated inflammatory markers, evidence of coagulopathy, acute gastrointestinal involvement, and myocardial injury associated with a SARS-CoV-2 infection, MIS-C was diagnosed. She received intravenous immunoglobulins (IVIG) 2 g/kg and steroids (dexamethasone 0.2 mg/kg). Hypoalbuminemia led to fluid overload after IVIG administration and was treated with furosemide. After six hours, urinary output and renal function improved.
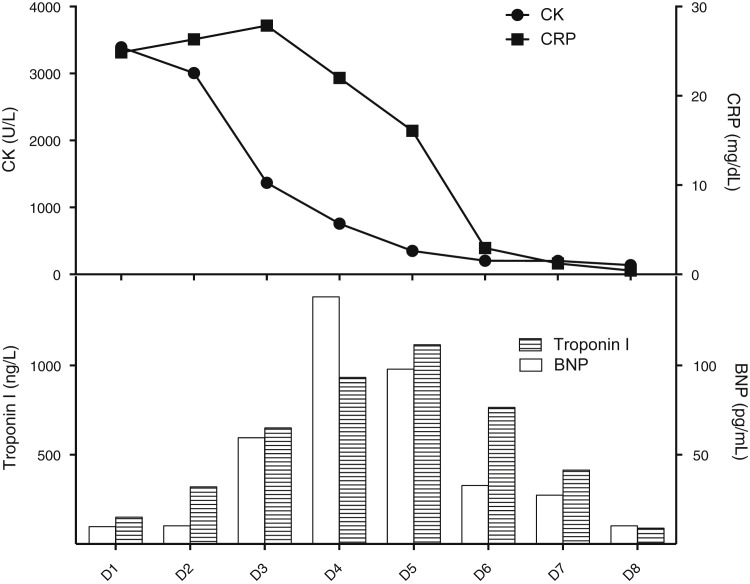
She continued to experience myalgia and profound asthenia for three days, while CK level gradually reduced over time along with CRP (Fig , top panel). Markers of myocardial injury increased over days and peaked later (brain natriuretic peptide: 1385 pg/ml on day four and troponin I: 111.6 ng/l on day five) (Fig, bottom panel) without evidence of ventricular dysfunction.
FIGURE.
Temporal relation among CK, CRP, and cardiac enzymes. CK and CRP gradually decrease with a similar temporal trend (top panel), while troponin I and BNP increase and peak later (bottom panel). Time is expressed on the X-axis as days (D, days). CK is expressed in IU/L, CRP in mg/dl, troponin I in ng/L, and BNP in pg/mL. BNP, brain natriuretic peptide; CK, creatinine kinase; CRP, C-reactive protein.
On day four, renal function and urine output normalized and the patient's muscle pain decreased. By the fifteenth day after her fever onset, her laboratory tests had normalized and she was discharged.
Discussion
SARS-CoV-2 binds angiotensin-converting enzyme 2 receptor expressed on skeletal muscle and causes renin-angiotensin down-regulation, directly affecting muscular integrity. Interestingly, some patients with COVID-19–related myositis have exhibited acute onset of severe muscle weakness, very high CK levels, elevated inflammatory markers, and clinical improvement after IVIG. These features highly resemble necrotizing autoimmune myositis, an inflammatory myopathy that has been described after viral infections.4
We hypothesize that SARS-CoV-2 could act as a trigger for myositis, inducing an immune T cell or macrophage-mediated response rather than a direct virus-induced cytotoxic damage, similarly to what was documented in the myocardium of adults with severe COVID-19 and in children with MIS-C.5 In our patient, markers of muscular damage seemed to reflect systemic inflammation rather than myocardial injury, thus indicating the skeletal muscle as a possible target of MIS-C. Second, myalgia and CK levels improved after the administration of IVIG and steroids, suggesting an immune-mediated mechanism similarly to the cases of necrotizing autoimmune myositis described for adults.
A dysregulation of the immune system and a hyperinflammatory response have been widely reported as mechanisms in the pathogenesis of MIS-C. Several autoantibodies against proteins involved in immune cell signaling, structural proteins of myocytes, and endothelial cells have been found, supporting an autoimmune mechanism in the pathogenesis of cardiovascular manifestations.6 The response to immunomodulating treatment could strengthen this hypothesis. Therefore, we hypothesize a similar mechanism acting in the skeletal muscle, highlighting awareness of this possible condition in children with MIS-C and the need to monitor CK levels.
Footnotes
Conflict of interest and source of funding statement: The authors declare no conflict of interest or financial disclosures concerning the materials or methods used in this study or the findings specified in this article.
Author contributions: All authors made substantial contributions to all of the following: (1) the conception and design of the study, acquisition of data, and analysis and interpretation of data; (2) drafting the article and revising it critically for important intellectual content; and (3) final approval of the version to be submitted.
References
- 1.Paliwal V.K., Garg R.K., Gupta A., Tejan N. Neuromuscular presentations in patients with COVID-19. Neurol Sci. 2020;41:3039–3056. doi: 10.1007/s10072-020-04708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gefen A.M., Palumbo N., Nathan S.K., Singer P.S., Castellanos-Reyes L.J., Sethna C.B. Pediatric COVID-19-associated rhabdomyolysis: a case report. Pediatr Nephrol. 2020;35:1517–1520. doi: 10.1007/s00467-020-04617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilpin S., Byers M., Byrd A., et al. Rhabdomyolysis as the initial presentation of SARS-CoV-2 in an adolescent. Pediatrics. 2020;147(3) doi: 10.1542/peds.2020-019273. e2020019273. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas M.C. Guillain-Barré syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e781. doi: 10.1212/NXI.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolhnikoff M., Ferreira Ferranti J., de Almeida Monteiro R.A., et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Heal. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consiglio C.R., Cotugno N., Sardh F., et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]