Introduction
The number of people impacted by eye disease is expected to increase in the next 3 decades with the aging of the US population. The total US economic burden of vision loss and eye disorders in 2013 was an estimated $139 billion.1 In 2015, the number of US adults aged 40 years and older that were visually impaired reached 3.22 million, which is expected to double by 2050.2 In the US, older adults, women, and African Americans endure a disproportionate amount of visual impairment (VI).2–4 Research is necessary to investigate how VI affects physical and mental health including vision-specific quality of life (VSQOL). Although it is established that VI reduces self-reported health outcomes, investigating specific domains of VSQOL impacted and the magnitude of effects for different US subpopulations may refine our knowledge of how people experience vision loss.
Population-based studies have not evaluated how VI—encompassing both visual field loss (VFL) and visual acuity (VA)—affects VSQOL in African Americans exclusively. But VFL has also been related to worse VSQOL in multicultural populations that include African Americans,5–9 as well as in population-based cohorts of Latinos.10–12 Existing literature has demonstrated that VFL is associated with reduced physical activity13 and travel from home14 as well as more frequent falls,15,16 hip fractures,17 and automobile accidents.18 Additional studies have found similar associations in glaucoma patients identified in clinics,7–9,19–23 but these results may not represent the impact VFL has on VSQOL in the broader population.
To the best of our knowledge this is the first study to assess how VFL impacts VSQOL in a population-based sample composed entirely of African Americans. We studied participants in the African American Eye Disease Study (AFEDS), a cohort of African Americans 40 years and older residing in Inglewood, California. We used item response theory (IRT) to propose loadings for survey items onto two domains of VSQOL—task and well-being. We hypothesized that VSQOL would be inversely related with mild VFL both as a continuous measure in the better-seeing eye (BSE) and as categories of bilateral VFL severity. Finally, we considered how more granular domains of VSQOL are impacted by VFL.
Methods
AFEDS is a population-based, cross-sectional cohort of 6,347 subjects aged 40 years and older residing in 32 US census tracts in the city of Inglewood, California. Data were collected from 2014–2018. A detailed description of data collection methods have been published elsewhere.24 In brief, eligible residents were identified by door-to-door census. Participants were interviewed in their homes and completed a comprehensive clinical eye examination at the local eye clinic. Home interviewing was conducted after informed consent to gather demographic factors and access to medical services. A comorbidity score was calculated as the sum of twelve self-reported medical conditions.25–27 Information on visual function and QOL was collected during the clinical eye exam. The University of Southern California Institutional Review Board approval was obtained prospectively before collecting data. All study procedures adhered to the recommendations of the Declaration of Helsinki.
VA was measured in each eye with presenting correction at 4 meters using standard Early Treatment Diabetic Retinopathy protocols with a modified distance chart illuminator (Precision Vision).28,29 VA loss was defined as presenting VA of 20/40 or worse based on the U.S. definition of VI. Visual fields for each eye were assessed using the Swedish Interactive Threshold Algorithm (SITA) Standard C24–2 test (Carl Zeiss Humphrey Field Analyzer II 750 Dublin, CA). VFL was measured as mean deviation (MD) in decibels (dB), where more negative scores indicated worse VFL. Unreliable measurements with more than 15% false negatives or false positives were excluded;30 fixation losses were not used as reliability criteria.31 A sensitivity analysis was performed to investigate the impact of reliability on visual field by relaxing cutoffs to 20% false positives and 25% false negatives.31 Continuous VFL was assessed as unilateral MD in the BSE, which has been shown to be as strong an indicator of VSQOL as integrated and binocular VFL.23,32 VFL categories were based on patterns of laterality (unilateral or bilateral) and severity (mild or moderate-to-severe).10–12,33 Participants were classified as having no VFL (MD > −2 dB in both eyes), unilateral mild VFL (−6 dB ≤ MD ≤ −2 dB and MD > −2 dB), unilateral moderate-to-severe VFL (MD < −6 and MD > −2 dB), bilateral mild VFL (−6 dB ≤ MD ≤ −2 dB; or MD < −6 dB and −6 dB ≤ MD ≤ −2), and bilateral moderate-to-severe VFL (MD < −6 dB).10,33 We collapsed clinical subdivisions of moderate and severe VFL into a single category due to low power in these groups individually; this is expected as both moderate and severe VFL are rare in the population, contrary to what is expected in the clinical setting.
The National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ-25) and the 12-Item Short Form Survey (SF-12) were administered by trained interviewers before the clinical examination. VSQOL was measured using the NEI-VFQ-25.34,35 CTT analysis of the NEI-VFQ-25 has been validated for various eye diseases in numerous populations.8,36–39 CTT was completed in the current analysis to allow comparisons with existing literature.10–12 Each item was scaled from 0 to 100, with 100 representing maximum VSQOL. 25 items were grouped into 11 vision-specific subscales; a CTT composite score was produced from the mean of all subscales. The NEI-VFQ-25 was also analyzed using the graded response model, a 2-parameter IRT model for ordinal items on a Likert scale.40,41 IRT models classified people with varying VSQOL scores along a linear continuum of item difficulty.42,43 IRT was used to produce two VSQOL composites from NEI-VFQ-25 items—task and well-being.44,45 The task composite score was calculated from 13 items belonging to subscales for vision-related role function, distance vision, driving difficulties, peripheral vision, near vision, and color vision. The well-being composite was calculated from 12 items for dependency, general vision, mental health, ocular pain, and social functioning. Task and well-being composite scores were calculated using SAS PROC IRT.46 Health-related quality of life (HRQOL) was measured as physical (PCS) and mental component summary (MCS) scores calculated for the standard US norm-based SF-12;47 a score of 50 (SD 10) was the average score among US adults.48
Differences in covariables were compared among non-excluded and excluded participants using Wilcoxon rank sum and Fisher’s exact tests. Continuous covariates were compared across VFL severity categories using analysis of variance, and categorical variables were compared using Bonferroni-adjusted Chi-squared tests; all covariables were evaluated using Tukey pairwise comparisons. Tests for trend were performed using the Wilcoxon rank sum test for continuous variables and the 2-sided, exact Cochran-Armitage test for categorical variables.
The generalized linear model was used to assess the relationship between VFL and VSQOL. Conceptual models of VI were developed during discussions with the AFEDS External Advisory Committee. Multivariable linear regression models were adjusted for age, number of comorbidities, sex (female), education (< 4 years of college), working status (unemployed), income (≤ $20,000), health and vision insurance (yes), visual acuity loss (20/40 or worse), and depression in the last 4 weeks (yes). Missing covariates were imputed by multiple imputation with chained equations (MICE).49 Locally weighted scatterplot smoothing (LOWESS) plots with 95% confidence intervals (CI) were produced for predicted VSQOL outcomes. A 5-unit change in the NEI-VFQ-25 has been associated with a 2-line deficit in VA which is considered a clinically important change in visual function;50 β coefficients from linear regression models were multiplied by 5 units of VSQOL to obtain the corresponding change in VFL. Analysis of covariance (ANCOVA) was used to calculate adjusted mean scores of VFL categories based on VFL severity categories. Effect sizes (ES) were calculated as the difference in adjusted mean scores of each VFL severity level from those without VFL divided by the SD for those without VFL. ES from 0.20–0.50 were considered small, 0.50–0.8 were medium, and ≥ 0.80 were large effects.51
All analyses were performed using SAS software 9.4 (SAS Institute, Cary, North Carolina, USA). Data visualization was produced using ggplot2 package (Hadley Wickham, Springer-Verlag New York) for R.
Results
Cohort Description
Of 7,957 identified as eligible, 6,347 (80.0%) participants were included in the final AFEDS cohort. The analytic cohort used for linear regression modeling was composed of 5,121 participants after excluding those with incomplete QOL scores or unreliable VFL measurements in both eyes (Supplementary Figure 1). Compared to those excluded (Supplementary Table 1), participants in the analytic cohort were younger (mean age 60.7 versus 62.0), had fewer comorbidities (mean 2.3 versus 2.7) and were less likely to be unemployed (54% versus 62%), to earn less than $20,000 per year (28% versus 39%), and to have VA loss (7% versus 17%) (P < 0.001). However, these differences were small for income (ES = 0.24) and VA loss (ES = 0.30) and negligible for age, comorbidities and unemployment (ES < 0.20).51 There were no statistically significant differences among the two groups in sex, education, health insurance, vision insurance, or depression status. The VFL severity cohort used for ANCOVA analyses was composed of 4,207 participants after further excluding participants with unreliable measurements in one eye (Supplementary Table 2).
More than 95% of the analytic cohort had complete sociodemographic and clinical characteristics, except for 994 (19%) participants with missing income (Table 1). The analytic cohort had a mean age of 60.7 years (SD 11.0 years) and 2.3 comorbidities on average (SD 1.9). Most were women (63%), did not complete college (65%), and had health (92%) and vision insurance (68%). Trends over VFL categories of increasing severity (P-trend < 0.01) were observed for older age, more comorbidities, lower income, greater unemployment, VA loss, and depression; there was also a trend with lower education (P = 0.04). There were no significant trends between VFL severity categories and sex, health insurance or vision insurance.
Table 1:
Sociodemographic and clinical characteristics of the AFEDS overall and across VFL severity categories
| Sociodemographic and Clinical Characteristics | Analytic Cohort (n = 5,121)* | Visual Field Loss Severity
Categories (n = 4,207)† |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No VFL (n = 2,348) | Unilateral | Bilateral | P-Value‡ | Trend Test⋄ | ||||||||||
| Mild (n = 848) | Moderate to Severe (n = 92) | Mild (n = 792) | Moderate to Severe (n = 127) | |||||||||||
|
| ||||||||||||||
| Age, Mean Years (SD) | 60.68 | (11.00) | 58.26 | (9.83)a | 60.92 | (10.78)b | 62.86 | (9.48)b,c | 64.25 | (12.10)c,d | 66.91 | (12.11)d | < 0.001 | < 0.001 |
| Comorbidities, Mean (SD)§ | 2.34 | (1.90) | 2.12 | (1.79)a | 2.39 | (1.86)b | 2.43 | (1.95)a,b,c | 2.58 | (1.96)b,c | 3.02 | (2.12)c | < 0.001 | < 0.001 |
| Female Sex | 3205 | (63%) | 1456 | (62%)a | 532 | (63%)a | 53 | (58%)a | 507 | (64%)a | 71 | (56%)a | 1.000 | 0.897 |
| Unemployed | 2698 | (54%) | 1050 | (46%)a | 458 | (56%)a,b | 51 | (57%)a,b | 486 | (63%)b | 99 | (79%)c | < 0.001 | < 0.001 |
| Income ≤ $20,000 | 1165 | (28%) | 436 | (23%)a | 186 | (27%)a,b | 18 | (24%)a | 223 | (35%)a,b | 36 | (39%)b | < 0.001 | < 0.001 |
| Education < 16 Years | 3260 | (65%) | 1452 | (63%)a,b | 546 | (66%)a,b | 50 | (56%)b | 517 | (67%)a,b | 89 | (71%)a | 0.484 | 0.038 |
| Health Insurance: Yes | 4592 | (92%) | 2090 | (91%)a | 764 | (92%)a | 83 | (92%)a | 717 | (92%)a | 119 | (95%)a | 1.000 | 0.078 |
| Vision Insurance: Yes | 3323 | (68%) | 1504 | (67%)a | 572 | (71%)a | 64 | (73%)a | 520 | (68%)a | 84 | (70%)a | 1.000 | 0.415 |
| Visual Acuity Loss: Yes∥ | 366 | (7%) | 71 | (3%)a | 48 | (6%)a,b | 7 | (8%)b,c | 114 | (14%)c | 34 | (27%)d | < 0.001 | < 0.001 |
| Depressed: Yes¶ | 303 | (6%) | 108 | (5%)a | 54 | (6%)a | 7 | (8%)a | 64 | (8%)a | 10 | (8%)a | 0.029 | < 0.001 |
AFEDS = African American Eye Disease Study; SD = Standard Deviation; VFL = Visual Field Loss; ANOVA = Analysis of Variance
Data are presented as mean (SD) for continuous variables (age and comorbidities); categorical variables are presented as frequency counts with percentages (%) of participants for each category of visual field loss (VFL) severity; percentages exclude participants with missing responses); the number missing is 144 (2.8%) for unemployment, 994 (19.4%) for income, 139 (2.7%) for education, 101 (2.0%) for insurance, 252 (4.9%) for vision insurance, and 6 (0.1%) for visual acuity loss.
The analytic cohort had reliable VF measurements in both eyes for 4,207 (81.3%) of participants and could be categorized into VFL severity categories. ANOVA least square means are shown across VFL severity categories with Tukey-Kramer-adjusted pairwise comparisons. Due to missing covariate data, not all VFL severity categories sum to the total (n = 4,207); the number missing is 108 (2.6%) for unemployment, 811 (19.3%) for income, 101 (2.4%) for education, 73 (1.7%) for insurance, 193 (4.6%) for vision insurance, and 2 (0.0%) for visual acuity loss.
P-values based on ANOVA type 3 sums of squares for continuous variables and χ2 tests for categorical variables (Bonferroni-adjusted for multiple comparisons). ANOVA revealed significant differences across the visual field loss categories for both age and number of comorbidities. Bonferroni-adjusted χ2 tests revealed significant differences for unemployment, annual income, visual acuity loss, and depression.
Number of self-reported comorbidities (diabetes, arthritis, stroke/brain hemorrhage, high blood pressure, angina, heart attack, heart failure, asthma, skin cancer, other cancer, back problems, hearing problems and other major health problems).
Visual Acuity Loss was defined as presenting visual acuity 20/40 or worse.
Depression was scored using the SF-12 item “Have you felt downhearted or blue a good bit of the time or more during the past 4 weeks?” Participants were considered depressed if they reported “A good bit of the time”, “Most of the time”, or “All of the time”.
Test for trend was performed by the nonparametric Wilcoxon rank sum test for continuous variables, and 2-sided, exact Cochran-Armitage test for categorical variables.
NEI-VFQ-25 Analysis
AFEDS participants tended to report high VSQOL, leading to a ceiling effect in the distribution of item responses on the NEI-VFQ-25 (Supplementary Figure 2).52 High measures of internal consistency were observed in IRT graded response models for both the task and well-being composites (Supplementary Table 3). Cronbach’s alpha was 0.86 for task and 0.78 for well-being indicating high inter-item correlation. Latent traits were unidimensional for both IRT composites; a single factor explained 66.9% of the variance for the task composite and 64.8% for the well-being composite. Test information curves demonstrated the NEI-VFQ-25 was most informative for task and well-being VSQOL scores two SDs below the mean.
Association of VSQOL and VFL
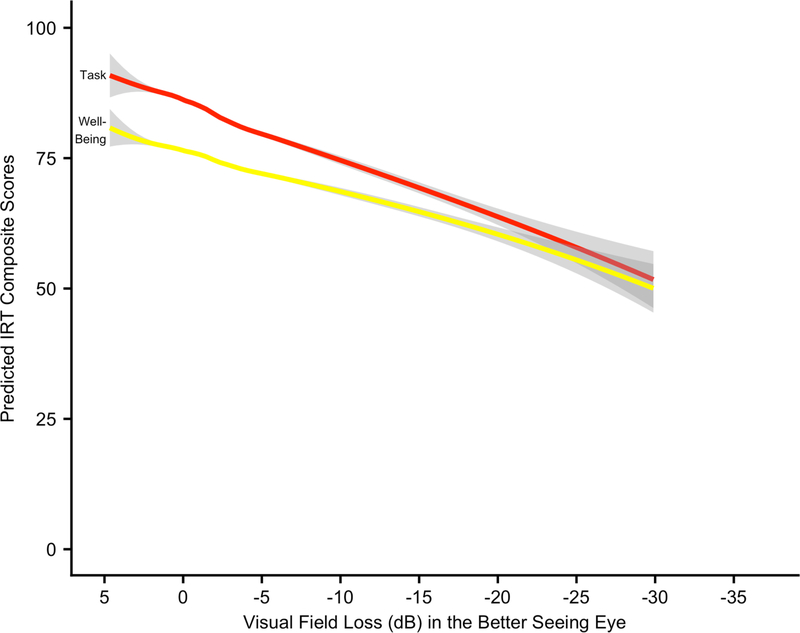
In this cohort of African Americans, VSQOL was inversely related to VFL after adjusting for covariables. LOWESS plots demonstrated strong, linear associations between predicted NEI-VFQ-25 IRT scores and VFL in the BSE (Figure 1). Predicted task VSQOL was greater than well-being for VFL ranging from none (0 dB) through severe (−20 dB), after which VSQOL scores were similar for both composites. Both IRT task (β = 0.80, 95% CI: 0.65, 0.95) and well-being (β = 0.54, 95% CI: 0.42, 0.67) composites were more strongly associated with VFL than the CTT composite (β = 0.38, 95% CI: 0.32, 0.44) (Table 2). VFL was most strongly associated with VSQOL subscales including driving difficulties (β = 0.83, 95% CI: 0.70, 0.97), general-vision (β = 0.58, 95% CI: 0.44, 0.71), near vision (β = 0.52, 95% CI: 42, 0.62), vision-related mental health (β = 0.48, 95% CI: 0.37, 0.58), and peripheral vision (β = 0.43, 95% CI: 0.34, 0.52). Clinically meaningful (5-point) differences in VSQOL were associated with decrements in VFL of 6.2 dB (95% CI: 5.3, 7.7) for task and 9.2 dB (95% CI: 7.5, 11.9) for well-being composites. Driving difficulties had a clinically important difference of 6.0 dB (95% CI: 5.2, 7.1), the strongest association with VFL of all QOL outcomes (Supplementary Figure 3). The SF-12 scores for HRQOL were either not significantly associated with VFL or the effects were negligible. Associations were attenuated for all QOL scores with VFL in the worse-seeing eye (Supplementary Table 4).
Figure 1:
LOWESS plot of predicted VSQOL IRT composite scores regressed on VFL in the BSE
LOWESS = Locally Weighted Scatterplot Smoothing; NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire 25-ltem; IRT = Item Response Theory; VFL = Visual Field Loss; MD = Mean Deviation; dB = Decibels; BSE = Better-Seeing Eye
The LOWESS smoothing parameter is 0.6. Gray bars represent 95% confidence limits of the predicted NEI-VFQ-25 IRT composite scores.
Linear regression models were adjusted for age, number of comorbidities, sex (female), education (< 4 years of college), working status (unemployed), income (≤ $20,000), has health insurance (yes), has vision insurance (yes), visual acuity loss (20/40 or worse), and depression (a good bit of the time or more in the last 4 weeks).
Table 2:
β coefficients from linear regression of VSQOL on VFL in the BSE adjusting for covariates
| Vision-Specific Quality of Life Measures | VFL in Better Seeing Eye | P-Value | |
|---|---|---|---|
| β coefficient (95% CI)* | MD (dB) of VFL Associated with 5-point Difference in QOL** | ||
|
| |||
| NEI-VFQ-25 | |||
| Item Response Theory | |||
| IRT Task Composite† | 0.80 (0.65, 0.95) | 6.2 | < 0.001 |
| IRT Well-Being Composite‡ | 0.54 (0.42, 0.67) | 9.2 | < 0.001 |
| Classical Test Theory | |||
| CTT Composite§ | 0.38 (0.32, 0.44) | 13.3 | < 0.001 |
| Driving Difficulties∥ | 0.83 (0.70, 0.97) | 6.0 | < 0.001 |
| General Vision | 0.58 (0.44, 0.71) | 8.7 | < 0.001 |
| Near Vision | 0.52 (0.42, 0.62) | 9.6 | < 0.001 |
| Vision-Related Mental Health | 0.48 (0.37, 0.58) | 10.5 | < 0.001 |
| Peripheral Vision | 0.43 (0.34, 0.52) | 11.7 | < 0.001 |
| Distance Vision | 0.38 (0.29, 0.46) | 13.3 | < 0.001 |
| Vision-Related Role Function | 0.34 (0.23, 0.46) | 14.5 | < 0.001 |
| Vision-Related Dependency | 0.30 (0.22, 0.38) | 16.8 | < 0.001 |
| Vision-Related Social Function | 0.23 (0.17, 0.28) | 22.1 | < 0.001 |
| Ocular Pain | 0.17 (0.07, 0.27) | 29.0 | 0.001 |
| Color Vision | 0.06 (0.01, 0.11) | 86.0 | 0.030 |
| General Health Item | |||
| General Health | 0.60 (0.41, 0.79) | 8.4 | < 0.001 |
| SF-12 | |||
| Mental Component Score | 0.08 (0.03, 0.13) | 62.1 | 0.001 |
| Physical Component Score | −0.01 (−0.05, 0.03) | 0.707 | |
VSQOL = Vision-Specific Quality of Life; VFL = Visual Field Loss; BSE = Better Seeing Eye; AFEDS = African American Eye Disease Study; 95% CI = 95% confidence interval; NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire 25-Item; MD = Mean Deviation; IRT = Item Response Theory; CTT = Classical Test Theory; SF-12 = 12-Item Short-Form Health Survey
VFL is presented as mean deviation score in decibels; vision-specific quality of life is assessed by the NEI-VFQ-25; and health-related quality of life is assessed by the SF-12. Data are presented as coefficient (95% CI). The SF-12 and NEI-VFQ-25 scores are adjusted for age, gender, education, employment status, income, acculturation, co-morbidities, health insurance, vision insurance, and visual acuity impairment.
Regression coefficients were transformed per 5-point difference in HRQOL score, a clinically significant difference in VSQOL score.
IRT Task Composite was calculated from a graded response theory model of 13 items from near vision, distance vision, driving, color vision, peripheral vision, and role difficulties subscales.
IRT Well-Being Composite was calculated from a graded response model of 12 items from general vision, dependency on others, mental health, ocular pain, and social functioning subscales.
Composite score is an un-weighted mean of the 12 subscale scores (excluding general health).
Scores could be generated for only 4,574 of the participants who reported that they were currently driving or had driven in the past.
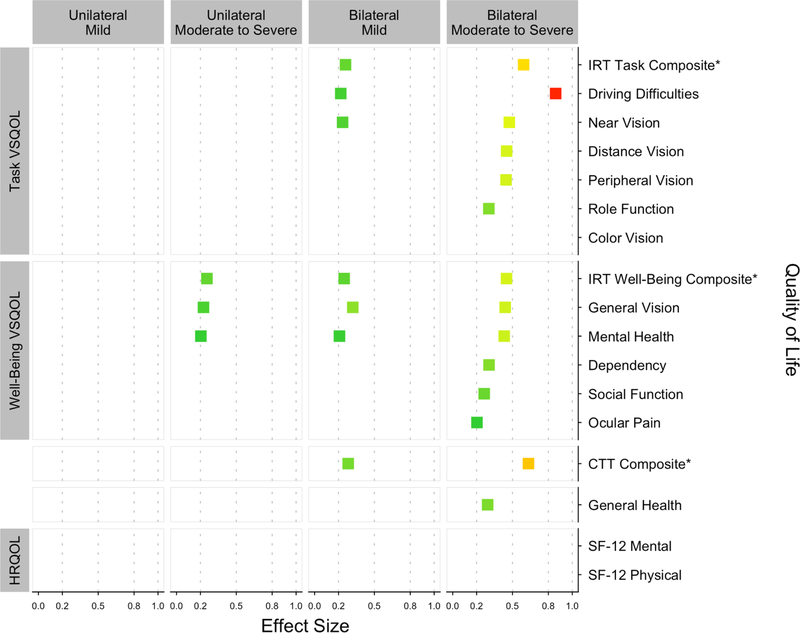
Only the worst VFL severity category had clinically meaningful differences in VSQOL compared to participants with normal vision (Table 3). VSQOL scores for those with bilateral moderate-to-severe VFL were meaningfully lower (≥ 5 points) for driving difficulties (−10.7), IRT task (−9.9), general vision (−6.5), IRT well-being (−6.2), general health (−6.3), near vision (−5.0), and mental health (−5.0). VSQOL was not meaningfully different for unilateral mild, unilateral moderate-to-severe, or bilateral mild VFL. VSQOL in participants with bilateral moderate-to-severe VFL had the largest difference in ES compared to those with normal vision (Figure 2). Driving difficulties (0.86) was the only comparison with a large ES (> 0.8). Similarly, the IRT task (0.59) and CTT overall (0.63) composites were the only domains with medium ES (0.5–0.8). Small ES (0.2–0.5) were observed in most remaining VSQOL outcomes including task domains [near vision (0.47), distance vision (0.45), peripheral vision (0.45), role-function (0.30)] and well-being domains [well-being IRT composite (0.45), general vision (0.44), mental health (0.43), dependency (0.30), social function (0.26), and ocular pain (0.20)]. ES were small in bilateral mild VFL for both IRT composites, general vision, near vision, driving difficulties, and mental health. ES were small in unilateral moderate-to-severe VFL for the well-being composite, general vision, and mental health. All comparisons in unilateral mild VFL were negligible (ES < 0.2). All comparisons of color vision and both HRQOL physical and mental component scores were also negligible.
Table 3:
ANCOVA comparing VSQOL scores across VFL severity categories adjusting for covariates
| Vision-Specific Quality of Life Measures Adjusted Mean Scores (SE)* | Visual Field Loss Severity
Categories (n = 4,207)‡ |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No VFL (n = 2,348) | Unilateral | Bilateral | P-Value§ | ||||||||
| Mild (n = 848) | Moderate to Severe (n = 92) | Mild (n = 792) | Moderate to Severe (n = 127) | ||||||||
|
| |||||||||||
| NEI-VFQ-25 | |||||||||||
| Item Response Theory | |||||||||||
| IRT Task Composite† | 86.8 | (0.3)a | 84.3 | (0.6)b | 84.9 | (1.7)a,b | 82.5 | (0.6)b | 76.9 | (1.5)c | < 0.001 |
| IRT Well-Being Composite‡ | 76.9 | (0.3)a | 75.2 | (0.5)b | 73.4 | (1.4)a,b,c | 73.5 | (0.5)c | 70.7 | (1.2)d | < 0.001 |
| Classical Test Theory | |||||||||||
| CTT Composite§ | 94.7 | (0.1)a | 93.7 | (0.2)b | 93.7 | (0.6)a,b | 93.0 | (0.2)b | 90.7 | (0.6)c | < 0.001 |
| Driving Difficulties∥ | 95.7 | (0.3)a | 93.5 | (0.4)b | 95.3 | (1.3)a,b | 93.0 | (0.5)b | 85.0 | (1.3)c | < 0.001 |
| General Vision | 75.1 | (0.3)a | 73.0 | (0.5)b | 71.7 | (1.5)a,b,c | 70.4 | (0.5)c | 68.6 | (1.3)d | < 0.001 |
| Near Vision | 95.2 | (0.2)a | 93.9 | (0.4)a,b | 94.3 | (1.1)a,b | 92.7 | (0.4)b | 90.2 | (0.9)c | < 0.001 |
| Vision-Related Mental Health | 91.4 | (0.2)a | 89.9 | (0.4)a,b | 89.1 | (1.2)a,b | 89.0 | (0.4)b | 86.4 | (1.0)c | < 0.001 |
| Peripheral Vision | 98.1 | (0.2)a | 96.9 | (0.3)b | 96.5 | (0.9)a,b | 96.6 | (0.3)b | 94.1 | (0.8)c | < 0.001 |
| Distance Vision | 97.4 | (0.2)a | 96.7 | (0.3)a,b | 96.9 | (0.9)a,b | 95.8 | (0.3)b | 93.5 | (0.8)c | < 0.001 |
| Vision-Related Role Function | 96.9 | (0.3)a | 96.2 | (0.4)a | 95.8 | (1.3)a | 95.8 | (0.5)a | 93.0 | (1.1)b | 0.010 |
| Vision-Related Dependency | 99.1 | (0.2)a | 98.4 | (0.3)a | 98.9 | (0.8)a | 98.1 | (0.3)a | 96.7 | (0.7)b | 0.001 |
| Vision-Related Social Function | 99.2 | (0.1)a | 98.6 | (0.2)a | 98.5 | (0.6)a | 98.5 | (0.2)a | 97.8 | (0.5)b | < 0.001 |
| Ocular Pain | 94.9 | (0.2)a | 94.3 | (0.4)a | 94.8 | (1.2)a | 93.9 | (0.4)a | 92.6 | (1.0)b | 0.058 |
| Color Vision | 99.2 | (0.1)a | 99.1 | (0.2)a | 99.5 | (0.6)a | 99.1 | (0.2)a | 98.9 | (0.5)b | 0.872 |
| General Health Item | |||||||||||
| General Health | 58.3 | (0.4)a | 56.5 | (0.7)a,b | 55.1 | (2.2)a,b,c | 55.0 | (0.8)b,c | 52.1 | (1.9)c | < 0.001 |
| SF-12 | |||||||||||
| Mental Component Score | 60.5 | (0.1)a | 59.7 | (0.2)a,b | 60.8 | (0.6)a,b | 59.9 | (0.2)b | 60.4 | (0.6)a,b | 0.008 |
| Physical Component Score | 39.8 | (0.1)a | 39.9 | (0.1)a | 39.8 | (0.5)a | 39.9 | (0.2)a | 39.0 | (0.4)a | 0.334 |
ANCOVA = analysis of covariance; VSQOL = Vision-Specific Quality of Life; VFL = Visual Field Loss; AFEDS = African American Eye Disease Study; NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire 25-Item; IRT = Item Response Theory; CTT = Classical Test Theory; SF-12 = 12-Item Short-Form Health Survey; MD = Mean Deviation
Adjusted mean (standard error) NEI-VFQ-25 and SF-12 scores. The covariates for adjustment include age, gender, education, employment status, income, comorbidities, health insurance, vision insurance, and presenting visual acuity 20/40 or worse.
VFL was classified as none (MD > −2), unilateral mild VFL (−6 ≤ MD ≤ −2 in the worse eye), unilateral moderate-to-severe VFL (MD < −6 in one eye, MD > −2 in the other eye), bilateral mild VFL (−6 ≤ MD ≤ −2 in both eyes, or −6 ≥ MD ≥ −2 in one eye, MD < −6 in the other eye), bilateral moderate-to-severe VFL (MD < −6 in the both eyes).
Analysis of covariance was used to compare adjusted mean scores across the different levels of unilateral and bilateral VFL. The P-value corresponds to the ANCOVA type III sums of squares f-test across the VFL groups. For each row, means with different letters (a–d) across the VFL categories are statistically significantly different from one another after adjusting for multiple comparisons using the Tukey-Kramer method (p < 0.05). ANCOVA revealed significant differences across the VFL categories for all VSQOL scales except ocular pain, color vision, and the physical component score of the SF-12.
Composite score is an un-weighted mean of 11 of the 12 NEI-VFQ-25 subscale scores (except general health).
Scores could be generated for only 3,808 of the participants who reported that they were currently driving or had driven in the past; the sample size was 2,199, 760, 84, 672, and 93 for the five VFL categories, respectively.
IRT Task Composite was calculated from a graded response model of 13 items from near vision, distance vision, driving, color vision, peripheral vision, and role difficulties subscales.
IRT Well-Being Composite was calculated from a graded response model of 12 items from general vision, dependency on others, mental health, ocular pain, and social functioning subscales.
Figure 2:
Effect sizes comparing VSQOL in each VFL severity category to those without VFL
VSQOL = Vision-Specific Quality of Life; AFEDS = African American Eye Disease Study; VFL = Visual Field Loss; CTT = Classical Test Theory; IRT = Item Response Theory; ANCOVA = Analysis of Covariance; ES = Effect Sizes; NEI-VFQ-25 = National Eye Institute Visual Function; SF-12 = 12-Item Short-Form Health Survey
ES below 0.20 are negligible and not shown. ES from 0.20 to less than 0.50 are considered small, 0.50 to less than 0.80 are medium, and 0.80 or more are large.
ES were calculated from ANCOVA models as the difference in adjusted mean QOL scores for each VFL severity category and the no VFL category, divided by the standard deviation of QOL score in the no VFL group.
ES are shown for the NEI-VFQ-25 CTT composite, the IRT task and well-being composites, all 11 CTT subscales, and the general health item; CTT subscales are grouped by task or well-being and ordered by descending ES in the bilateral moderate/severe comparison. The SF-12 component scores are also shown.
VFL severity was stratified into five categories: no VFL (mean deviation [MD] > 2 decibels [dB] in both eyes), unilateral mild VFL (−6 dB <MD< −2 dB in the worse eye); bilateral mild VFL ( 6 dB < MD < 2 dB in both eyes; unilateral moderate-to-severe VFL (MD<−6 dB in one eye, MD > 2 dB in the other eye; or 6 dB < MD < 2 dB in one eye, MD < 6 dB in the other eye), and bilateral moderate-to-severe VFL (MD < 6 dB in both eyes).
*NEI-VFQ-25 item response theory and classical test theory composite scores are marked for emphasis.
||Scores could be generated for only 4,574 of the participants who reported that they were currently driving or had driven in the past.
Relaxing the VFL reliability cutoffs resulted in 235 additional participants (3.7% of the AFEDS) being included in the sensitivity analysis (Supplementary Figure 4). Beta estimates from the linear regressions of VSQOL on VFL in the BSE differed in magnitude by less than 15%, and least square means in the ANCOA varied by less than 5%. The singular exception was the association between the IRT task composite and VFL in the BSE which was reduced from β = 0.80 (95% CI: 0.65–0.95) to β = 0.6 (95% CI: 0.49–0.72).
Discussion
In this study of African Americans, VFL was associated with lower VSQOL. NEI-VFQ-25 survey items were analyzed using IRT analysis to generate two summary composites of VSQOL: completing vision-specific daily tasks and vision-related socioemotional well-being. Associations between VFL and VSQOL were stronger for both IRT composites compared to the traditional CTT analysis, which was used to produce a single composite from all 25 items of the NEI-VFQ. The average VSQOL score for participants in AFEDS with normal vision was 10-points lower for socioemotional well-being composite compared to the daily task; however, the inverse relationship between decibels of VFL and VSQOL was stronger for vision-specific daily task composite. More precisely, a 5-point difference of VSQOL in the task composite was associated with mild-to-moderate VFL (6.2 dB; 95% CI 5.3–7.7), but the same difference in the well-being composite was observed only after reaching moderate VFL (9.2 dB; 95% CI 7.5–11.9). Furthermore, VFL had the greatest impact on several subscales contributing to task VSQOL—driving difficulties, near vision, and peripheral vision—and to well-being VSQOL—general vision and mental health.
We found a dose-response relationship by severity and laterality of VFL and VSQOL, with a larger association for vision-specific task compared to well-being. The largest differences in task VSQOL were among participants with bilateral moderate-to-severe VFL compared to those with normal vision. But only small differences in well-being VSQOL were observed for all severity levels of VFL. Clinicians should be aware that patients with worse than 6 dB of VFL in both eyes may be unable to complete visual tasks, which may limit independent mobility due to diminished driving ability. Those with bilateral VFL of 2–6 dB may experience greater reductions in their vision-related mental health as well as their ability to drive and read up close. Furthermore, patients with worse than 6 dB of unilateral VFL may be more worried, experience frustration, and feel uncertainty due to their vision before experiencing deficits in their ability to complete vision-related tasks.
Despite known disparities in visual function, this is the first population-based study to assess the relationship between VFL and VSQOL in a large, population-based sample of only African Americans. Several population-based studies have found that worse VFL is associated with lower VSQOL in multiethnic cohorts that include African Americans,5,6,53,54 but existing studies used exclusively CTT which may limit interpretations.44,52 Using available data, population-based investigations of diverse populations found that African Americans were disproportionately impacted by VFL. In the National Health and Nutrition Examination Survey, a higher percentage of participants reported difficulty with visual function based on the NEI-VFQ-25 with worse VFL, and African Americans were three times more likely to have severe VFL compared to non-Hispanic Whites.6 Similar to the present study, driving difficulties during the day was most strongly associated with VFL. In the Salisbury Eye Evaluation Study (SEES), a 2-fold reduction in central visual field was associated with 1.37 (95% CI 1.19–1.58) greater odds of scoring in the worst tertile of VSQOL;54 African Americans had worse VFL compared to non-Hispanic Whites across all age strata. In a population of African descent outside the US, the Barbados Eye Study (BES) found reduced VSQOL was associated with primary open angle glaucoma (OAG), a common cause of VFL.55 Additional population-based studies that have evaluated the impact of VFL on VSQOL or function have focused on non-Hispanic White5,6,17,53,54,56–60 and more recently on Latino populations10–12.
Population-based, cross cultural investigations are needed to better understand whether race/ethnicity mediates the effect of VFL on VSQOL. Although harmonized methods across existing studies are necessary for valid comparisons, the Los Angeles Latino Eye Study (LALES) reported on the linear relationship of VFL with continuous measures of vision-specific functional domains from the NEI-VFQ-25; mild VFL (5 dB for CTT composite) was associated with a clinically meaningful (5-point) difference in NEI-VFQ-25 CTT composite score.10 In African Americans in the present study, a larger change in VF (5–8 dB for task and 7–12 dB for well-being VSQOL) was necessary to observe the same 5-point difference in the NEI-VFQ-25 CTT composite score. However, the current IRT models for evaluating the vision-specific daily task and emotional well-being composite scores were not completed for the LALES. A pooled analysis with common statistical methods and confounder adjustment may elucidate whether race and ethnicity interact with the association of VFL and VSQOL.
African Americans endure a disproportionate burden of OAG compared to non-Hispanic Whites.3,4 Few population-based studies have evaluated the association between VFL and VSQOL in glaucoma patients of African descent. The SEES found African American glaucoma patients were twice as likely as non-Hispanic Whites to report worse VSQOL.5 In the BES, all participants were of African descent and 19% were diagnosed with OAG.55 Glaucoma patients had significantly lower NEI-VFQ-25 subscale scores for vision-specific mental health, social functioning, and distance vision, however models were not adjusted for VFL. Additionally, a clinic-based, multi-center study found 8 subscales of the NEI-VFQ-25 were negatively associated by VFL in glaucoma patients, a third of whom were African American;7 driving difficulties had the strongest linear association with VFL. Another study in Alabama was two-thirds African American and observed worsening VFL was associated with a decrease in most NEI-VFQ-25 subscales.8 Interestingly, findings were similar for both White and African American patients. Further research should investigate whether racial/ethnic disparities in eye disease include self-reported VSQOL outcomes.
Our findings may be limited by restricting to only the most reliable VF measurements. We performed a sensitivity analysis by raising reliability thresholds to 20% false positives and 25% false negatives according to evidence-based cutoffs for glaucoma patients.31 We observed minimal differences for all associations between VFL and VSQOL except for a 25% reduction in the relationship between VFL and completing vision-related tasks. Using more restrictive cutoffs for reliability of VF measurements led to the exclusion of 3.7% of AFEDS participants, some of whom were unable to reliably complete the Humphrey Field Analyzer exam due to advanced disease. This resulted in a stronger underlying association between VFL and completing visual tasks. However, the vast majority of findings were similar for either definition of VF reliability cutoffs.
Findings in the AFEDS may be generalizable to African Americans living throughout the USA, but comparisons may be limited for communities of lower socioeconomic status. The AFEDS cohort was highly educated, had greater income, and 90% had access to health insurance compared to African Americans in the general US population. However, baseline assessment found the remaining sociodemographics in the AFEDS cohort were similar to African Americans in Los Angeles, California, and the country overall.24
In this population-based sample of African Americans, we found that 6 to 9 dB of VFL in the BSE was associated with a 5-point difference in participants’ abilities to complete vision related tasks and their socioemotional well-being. VFL had the greatest impact on driving-related VSQOL: a 6 dB difference of VFL in the BSE was associated with a 5-point change in self-reported driving ability. Among participants with moderate-to-severe VFL in both eyes, we observed medium-to-large detriments in ability to complete daily vision tasks, and small losses of vision-related socioemotional well-being. As VFL worsens, providers should be aware of moderate reductions in well-being and mental health, which may precipitate greater inability to complete daily activities involving peripheral, distance, and near vision. Longitudinal studies of VFL in population-based samples of African Americans are necessary to assess whether patterns observed in this cross-sectional data are predictive of individuals’ experiences.
Supplementary Material
Acknowledgements
Funding/Support
The funding organizations had no role in the design or conduct of this research.
Design and conduct of the study
Collection, management, analysis, and interpretation of the data
NIH National Eye Institute Grant U10 EY-023575
Unrestricted grant from the Research to Prevent Blindness, New York, New York
NIH National Institute on Aging Grant T32 AG000037
Preparation, review, or approval of the manuscript
NIH National Institute of Environmental Health Sciences Grant T32 ES013678
Other Acknowledgements
The authors would like to thank the African American Eye Disease Study External Advisory Committee for their advice and contributions: M. Roy Wilson, MD, MS (Chair); Julia A. Haller, MD; Helen Hazuda, PhD; Eve J. Higginbotham, SM, MD; Joanne Katz, ScD; Maryann Redford, DDS, MPH; and Xinzhi Zhang, MD, PhD, FACE, FRSM. The authors would also like to acknowledge support of the analysis for this project from Wendy J. Mack, PhD; Lourdes A. Baezconde-Garbanati, PhD, MPH; and Eileen M. Crimmins, PhD.
Abbreviations
- (AFEDS)
African American Eye Disease Study
- (BSE)
Better Seeing Eye
- (CTT)
Classical Test Theory
- (ES)
Effect Sizes
- (GRM)
Graded Response Model
- (IRT)
Item Response Theory
- (LOWESS)
Locally Weighted Scatterplot Smoothing
- (LALES)
Los Angeles Latino Eye Study
- (NEI-VFQ-25)
National Eye Institute Visual Functioning Questionnaire-25
- (OAG)
Open Angle Glaucoma
- (PRO)
Patient Reported Outcome
- (SEES)
Salisbury Eye Evaluation Study
- (VA)
Visual Acuity
- (VFL)
Visual Field Loss
- (VSQOL)
Vision Specific Quality of Life
- (WSE)
Worse Seeing Eye
- (SF-12)
12-Item Short-Form Health Survey
Footnotes
Financial Disclosures
Dominic Grisafe: None; Rohit Varma: Allegro Inc. Code C (Consultant), Bausch Health Companies Inc. Code C (Consultant), Allergan Code C (Consultant), MHR Vision Code I (Personal Financial Interest); Bruce Burkemper: None; Benjamin Xu: Heidelberg Code C (Consultant); Mina Torres: None; Alicia Fairbrother-Crisp: None; Cecilia Patino: None; Roberta McKean-Cowdin: None
The African American Eye Disease Study Group, University of Southern California, Los Angeles, CA: Rohit Varma, MD, MPH; Roberta McKean-Cowdin, PhD; Dominic J. Grisafe II, PhD; Mina Torres, MS; Benjamin Xu, MD, PhD; Grace Richter, MD; Alicia Fairbrother-Crisp, MPH; Farzana Choudhury MBBS, MS, PhD; Xuejuan Jiang, PhD; Bruce Burkemper, PhD, MS; Tengiz Adamashvili; Carlos Lastra, MD; Elizabeth Corona; YuPing Wang, COT; Jacqueline Douglass; Jaimie Barrera; Judith Linton; Kisha Milo.
Department of Ophthalmology, Battelle Survey Research Center, St. Louis, MO: Lisa John, PhD; Nicole Weinstein, MSW; Natasha Van Leeuwen; James Clark; Sandra Ramirez.
Singapore National Eye Centre, Ocular Reading Center: Tien Wong, MD, PhD; Soundaram Jaganathan; Haslina Hamzah.
Supplementary Materials are available at AJO.com
References
- 1.Wittenborn J, Rein D. Cost of Vision Problems: The Economic Burden of Vision Loss and Eye Disorders in the United States. University of Chicago; 2013. http://www.norc.org/Research/Projects/Pages/the-economic-burden-of-vision-loss-and-eye-disorders-in-the-united-states.aspx [Google Scholar]
- 2.Varma R, Vajaranant TS, Burkemper B, et al. Visual Impairment and Blindness in Adults in the United States: Demographic and Geographic Variations from 2015 to 2050. JAMA Ophthalmol. 2016;134(7):802–809. doi: 10.1001/jamaophthalmol.2016.1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Klein BEK. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF5–ORSF13. doi: 10.1167/iovs.13-12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DS, Jampel HD, Muñoz B, West SK. The prevalence of open-angle glaucoma among blacks and whites 73 years and older: the Salisbury Eye Evaluation Glaucoma Study. Arch Ophthalmol Chic Ill 1960. 2006;124(11):1625–1630. doi: 10.1001/archopht.124.11.1625 [DOI] [PubMed] [Google Scholar]
- 5.Freeman EE, Munoz B, West SK, Jampel HD, Friedman DS. Glaucoma and quality of life: the Salisbury Eye Evaluation. Ophthalmology. 2008;115(2):233–238. doi: 10.1016/j.ophtha.2007.04.050 [DOI] [PubMed] [Google Scholar]
- 6.Qiu M, Wang SY, Singh K, Lin SC. Association between visual field defects and quality of life in the United States. Ophthalmology. 2014;121(3):733–740. doi: 10.1016/j.ophtha.2013.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez P, Wilson MR, Johnson C, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997;115(6):777–784. [DOI] [PubMed] [Google Scholar]
- 8.Ringsdorf L, McGwin G, Owsley C. Visual field defects and vision-specific health-related quality of life in African Americans and whites with glaucoma. J Glaucoma. 2006;15(5):414–418. doi: 10.1097/01.ijg.0000212252.72207.c2 [DOI] [PubMed] [Google Scholar]
- 9.Parrish RK, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol. 1997;115(11):1447–1455. [DOI] [PubMed] [Google Scholar]
- 10.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143(6):1013–1023. doi: 10.1016/j.ajo.2007.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patino CM, Varma R, Azen SP, et al. The impact of change in visual field on health-related quality of life the los angeles latino eye study. Ophthalmology. 2011;118(7):1310–1317. doi: 10.1016/j.ophtha.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(6):941–948.e1. doi: 10.1016/j.ophtha.2007.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Landingham SW, Willis JR, Vitale S, Ramulu PY. Visual field loss and accelerometer-measured physical activity in the United States. Ophthalmology. 2012;119(12):2486–2492. doi: 10.1016/j.ophtha.2012.06.034 [DOI] [PubMed] [Google Scholar]
- 14.Ramulu PY, Hochberg C, Maul EA, Chan ES, Ferrucci L, Friedman DS. Glaucomatous visual field loss associated with less travel from home. Optom Vis Sci. 2014;91(2):187–193. doi: 10.1097/opx.0000000000000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramulu PY, Mihailovic A, West SK, Gitlin LN, Friedman DS. Predictors of Falls per Step and Falls per Year At and Away From Home in Glaucoma. Am J Ophthalmol. 2019;200:169–178. doi: 10.1016/j.ajo.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patino CM, McKean-Cowdin R, Azen SP, Allison JC, Choudhury F, Varma R. Central and peripheral visual impairment and the risk of falls and falls with injury. Ophthalmology. 2010;117(2):199–206.e1. doi: 10.1016/j.ophtha.2009.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivers RQ, Cumming RG, Mitchell P, Simpson JM, Peduto AJ. Visual risk factors for hip fracture in older people. J Am Geriatr Soc. 2003;51(3):356–363. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CA, Keltner JL. Incidence of visual field loss in 20,000 eyes and its relationship to driving performance. Arch Ophthalmol. 1983;101(3):371–375. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros FA, Gracitelli CP, Boer ER, Weinreb RN, Zangwill LM, Rosen PN. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology. 2015;122(2):293–301. doi: 10.1016/j.ophtha.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe RY, Diniz-Filho A, Costa VP, Gracitelli CP, Baig S, Medeiros FA. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology. 2016;123(3):552–557. doi: 10.1016/j.ophtha.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Gestel A, Webers CA, Beckers HJ, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye Lond. 2010;24(12):1759–1769. doi: 10.1038/eye.2010.133 [DOI] [PubMed] [Google Scholar]
- 22.Lisboa R, Chun YS, Zangwill LM, et al. Association between rates of binocular visual field loss and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol. 2013;131(4):486–494. doi: 10.1001/jamaophthalmol.2013.2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun YS, Lee DI, Kwon J, Park IK. Comparison of Impact of Monocular and Integrated Binocular Visual Fields on Vision-related Quality of Life. J Glaucoma. 2017;26(3):283–291. doi: 10.1097/ijg.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 24.McKean-Cowdin R, Fairbrother-Crisp A, Torres M, et al. The African American Eye Disease Study: Design and Methods. Ophthalmic Epidemiol. 2018;25(4):306–314. doi: 10.1080/09286586.2018.1454965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108(10):1893–1900; discussion 1900–1901. doi: 10.1016/s0161-6420(01)00754-0 [DOI] [PubMed] [Google Scholar]
- 26.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x [DOI] [PubMed] [Google Scholar]
- 27.Globe DR, Varma R, Torres M, et al. Self-reported comorbidities and visual function in a population-based study: the Los Angeles Latino Eye Study. Arch Ophthalmol. 2005;123(6):815–821. doi: 10.1001/archopht.123.6.815 [DOI] [PubMed] [Google Scholar]
- 28.Ferris FL, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: Guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103(1):181–182. [DOI] [PubMed] [Google Scholar]
- 29.Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 30.Heijl A, Patella VM, Bengtsoon B. The Field Analyzer Primer: Effective Perimetry. Carl Zeiss Meditec Incorporated; 2012. [Google Scholar]
- 31.Yohannan J, Wang J, Brown J, et al. Evidence-based Criteria for Assessment of Visual Field Reliability. Ophthalmology. 2017;124(11):1612–1620. doi: 10.1016/j.ophtha.2017.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arora KS, Boland MV, Friedman DS, Jefferys JL, West SK, Ramulu PY. The Relationship between Better-Eye and Integrated Visual Field Mean Deviation and Visual Disability. Ophthalmology. 2013;120(12):2476–2484. doi: 10.1016/j.ophtha.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cello KE, Nelson-Quigg JM, Johnson CA. Frequency doubling technology perimetry for detection of glaucomatous visual field loss. Am J Ophthalmol. 2000;129(3):314–322. [DOI] [PubMed] [Google Scholar]
- 34.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116(11):1496–1504. [DOI] [PubMed] [Google Scholar]
- 35.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. [DOI] [PubMed] [Google Scholar]
- 36.Baker RS, Bazargan M, Calderon JL, Hays RD. Psychometric performance of the National Eye Institute visual function questionnaire in Latinos and non-Latinos. Ophthalmology. 2006;113(8):1363–1371. doi: 10.1016/j.ophtha.2006.01.073 [DOI] [PubMed] [Google Scholar]
- 37.Paz SH, Globe DR, Wu J, Azen SP, Varma R. Relationship between self-reported depression and self-reported visual function in Latinos. Arch Ophthalmol. 2003;121(7):1021–1027. doi: 10.1001/archopht.121.7.1021 [DOI] [PubMed] [Google Scholar]
- 38.Clemons TE, Chew EY, Bressler SB, McBee W. National Eye Institute Visual Function Questionnaire in the Age-Related Eye Disease Study (AREDS): AREDS Report No. 10. Arch Ophthalmol. 2003;121(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen CG, Rudnicka AR, Smeeth L, Evans JR, Wormald RP, Fletcher AE. Is the NEI-VFQ-25 a useful tool in identifying visual impairment in an elderly population? BMC Ophthalmol. 2006;6:24. doi: 10.1186/1471-2415-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang S, Wang C, Weiss DJ. Sample Size Requirements for Estimation of Item Parameters in the Multidimensional Graded Response Model. Front Psychol. 2016;7:109. doi: 10.3389/fpsyg.2016.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edelen MO, Reeve BB. Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Qual Life Res. 2007;16 Suppl 1:5–18. doi: 10.1007/s11136-007-9198-0 [DOI] [PubMed] [Google Scholar]
- 42.Andrich D. Rating scales and Rasch measurement. Expert Rev Pharmacoecon Outcomes Res. 2011;11(5):571–585. doi: 10.1586/erp.11.59 [DOI] [PubMed] [Google Scholar]
- 43.Streiner DL, Norman GR, Cairney J. Health Measurement Scales: A Practical Guide to Their Development and Use. 5th ed. Oxford University Press; 2015. [Google Scholar]
- 44.Pesudovs K, Gothwal VK, Wright T, Lamoureux EL. Remediating serious flaws in the National Eye Institute Visual Function Questionnaire. J Cataract Refract Surg. 2010;36(5):718–732. doi: 10.1016/j.jcrs.2009.11.019 [DOI] [PubMed] [Google Scholar]
- 45.Marella M, Pesudovs K, Keeffe JE, O’Connor PM, Rees G, Lamoureux EL. The psychometric validity of the NEI VFQ-25 for use in a low-vision population. Invest Ophthalmol Vis Sci. 2010;51(6):2878–2884. doi: 10.1167/iovs.09-4494 [DOI] [PubMed] [Google Scholar]
- 46.Cole K. Rasch Model Calibrations with SAS PROC IRT and WINSTEPS. J Appl Meas. 2019;20(1):27–45. [PubMed] [Google Scholar]
- 47.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 48.Loriaut P, Boyer P, Massin P, Cochereau I. Visual impairment and hip fractures: a case-control study in elderly patients. Ophthalmic Res. 2014;52(4):212–216. doi: 10.1159/000362881 [DOI] [PubMed] [Google Scholar]
- 49.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple Imputation by Chained Equations: What is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Globe DR, Wu J, Azen SP, Varma R, Los Angeles Latino Eye Study G. The impact of visual impairment on self-reported visual functioning in Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2004;111(6):1141–1149. doi: 10.1016/j.ophtha.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 51.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed.; 1988. https://books.google.com/books/about/Statistical_Power_Analysis_for_the_Behav.html?id=cIJH0lR33bgC [Google Scholar]
- 52.Petrillo J, Cano SJ, McLeod LD, Coon CD. Using classical test theory, item response theory, and Rasch measurement theory to evaluate patient-reported outcome measures: a comparison of worked examples. Value Health. 2015;18(1):25–34. doi: 10.1016/j.jval.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 53.Valbuena M, Bandeen-Roche K, Rubin GS, Munoz B, West SK. Self-reported assessment of visual function in a population-based study: the SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1999;40(2):280–288. [PubMed] [Google Scholar]
- 54.Rubin GS, Bandeen-Roche K, Huang GH, et al. The association of multiple visual impairments with self-reported visual disability: SEE project. Invest Ophthalmol Vis Sci. 2001;42(1):64–72. [PubMed] [Google Scholar]
- 55.Wu S-Y, Hennis A, Nemesure B, Leske MC, Barbados Eye Studies Group. Impact of glaucoma, lens opacities, and cataract surgery on visual functioning and related quality of life: the Barbados Eye Studies. Invest Ophthalmol Vis Sci. 2008;49(4):1333–1338. doi: 10.1167/iovs.07-1252 [DOI] [PubMed] [Google Scholar]
- 56.Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998;46(1):58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x [DOI] [PubMed] [Google Scholar]
- 57.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Risk factors for fractures of the wrist, shoulder and ankle: the Blue Mountains Eye Study. Osteoporos Int. 2002;13(6):513–518. doi: 10.1007/s001980200063 [DOI] [PubMed] [Google Scholar]
- 58.Freeman EE, Munoz B, Turano KA, West SK. Measures of visual function and their association with driving modification in older adults. Invest Ophthalmol Vis Sci. 2006;47(2):514–520. doi: 10.1167/iovs.05-0934 [DOI] [PubMed] [Google Scholar]
- 59.Kaleem MA, Munoz BE, Munro CA, Gower EW, West SK. Visual characteristics of elderly night drivers in the Salisbury Eye Evaluation Driving Study. Invest Ophthalmol Vis Sci. 2012;53(9):5161–5167. doi: 10.1167/iovs.12-9866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao P, Munoz B, Turano K, Munro C, West SK. The decline in attentional visual fields over time among older participants in the Salisbury Eye Evaluation Driving Study. Invest Ophthalmol Vis Sci. 2013;54(3):1839–1844. doi: 10.1167/iovs.11-8874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.