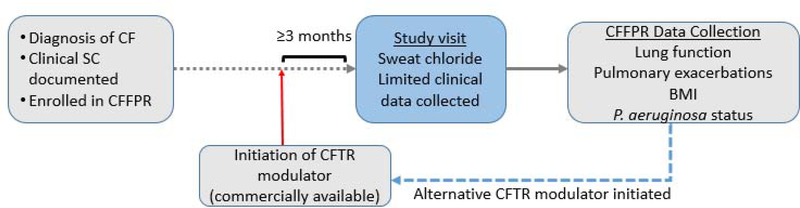
Figure 3. CHEC-SC Study Design.
Individuals with CF are eligible if on a commercially available CFTR modulator for at least three months. A single study visit is completed with sweat chloride measurement and data collection. Data from the CFF Patient Registry is collected to determine long term outcomes. Patients may re-enroll if an alternative CFTR modulator is initiated.